This study aimed to compare the efficacy of two sustained-release formulation of artificial tear drops.
Patients and methodsThis is a randomized patient-masked clinical trial, a total 88 patients into two group A (n=41; with single dose of artificial tear, containing dextran 70, 1mg/ml and hypromellose, 3mg/ml hydroxypropyl methylcellulose (HPMC) and group B (n=47; with multidose of artificial tear, containing 0.3g HPMC and 0.1g of dextran 70, with 0.01% benzalkonium chloride (BAK) as preservative) were completed the study. The ocular surface disease index (OSDI) questionnaire, tear break up time (TBUT), corneal and conjunctival staining and Schirmer test, were performed. Repeated measures ANOVA was used to assess the differences among the two products. A p-value less than 0.05 was considered significant.
ResultsThe mean of age of the participants in the Group A and B was 44.08±6.29 (range, 33–58 years) years and 45.83±8.42 (31–60 years), respectively. In comparing two groups before the intervention, the OSDI scores, the TBUT scores, the conjunctival and corneal staining scores and the Schirmer scores did not show statistically significant differences (p=0.339, p=0.640, p=0.334, p=0.807 and p=0.676, respectively). After 4 weeks, the OSDI scores, conjunctival and corneal staining scores showed improvement in compare to those before the intervention (p<0.001). But, the differences for the Schirmer test score and TBUT score was not significant (p=0.115, p=0.013, respectively).
ConclusionOur outcomes indicated that improvement occurred with use of both products but there was no statistically significant difference between them.
El objetivo de este estudio fue comparar la eficacia de dos fórmulas de lágrimas artificiales de liberación sostenida.
Pacientes y MétodosEnsayo clínico aleatorizado y enmascarado para el paciente, se incluyó a un total de 88 pacientes distribuidos en dos grupos: el grupo A (n=41; con una dosis única de lágrima artificial con contenido de Dextran 70,1mg/ml e hipromelosa, 3mg/ml hidroxipropil metilcelulosa (HPMC), y el grupo B (n=47; con multidosis de lágrima artificial, con contenido de 0,3g HPMC y 0,1g de Dextran 70, y 0,01% de cloruro de benzalconio (BAK) como conservante). Se realizaron las siguientes pruebas: cuestionario del índice de enfermedad de la superficie ocular (OSDI), tear break-up time (TBUT), tinción corneal y conjuntival y prueba de Schirmer. Para el análisis estadístico se utilizó ANOVA para mediciones repetidas, a fin de evaluar las diferencias entre los dos productos. Se consideró significativo un valor p inferior a 0,05.
ResultadosLa media de edad de los participantes de los grupos A y B fue de 44,08±6,29 (rango de 33 a 58 años) y 45,83±8,42 (de 31 a 60 años), respectivamente. Al comparar los dos grupos antes de la intervención, las puntuaciones OSDI, TBUT, las de tinción conjuntival y corneal, y las de la prueba de Schirmer no reflejaron diferencias estadísticamente significativas (p=0,339, p=0,640, p=0,334, p=0,807 y p=0,676, respectivamente). Transcurridas cuatro semanas, las puntuaciones OSDI y las de tinción conjuntival y corneal reflejaron una mejora en comparación a las puntuaciones anteriores a la intervención (p<0,001). Pero las diferencias en cuanto a las puntuaciones de la prueba de Schirmer y TBUT no fueron significativas (p=0,115, p=0,013, respectivamente).
ConclusiónNuestros resultados indican que se produjo una mejora con el uso de ambos productos, pero que no se produjo una diferencia estadísticamente significativa entre ambos.
Dry eye syndrome (DES) is a multifactorial disease of the ocular surface. Rapid evaporation of tear film, inadequate production of tears, and inflammation of the ocular surface are among the causes of this syndrome. This condition can result in the ocular symptoms of foreign body sensation, redness, and discomfort, as well as the signs of surface damage in the cornea and conjunctiva, all leading to detrimental visual performance.1–4 DES is a common problem worldwide and can reduce the working efficiency of an individual. Dry eye is therefore a frequent complaint that patient present to eye care clinics. Common patient's complaints related to dry eye include reduced vision, difficulty reading, difficulty driving at night and difficulty doing computer work.5 A key principle for the management of dry eye disease is augmentation of the tear film through the topical administration of artificial tear substitutes. These products enhance tear stability thus reducing loss by evaporation; this, in turn, helps to retain moisture in the eye and relieve the chronic ocular inflammation associated with dry eyes. Artificial tear substitutes help to reduce patient discomfort, improve quality of life and reduce the risk of damage to the corneal epithelium.6 Artificial tears are among the first line of therapy in management of DES.8 They may be used along with other treatments such oral omega-3 essential fatty acid supplements, mucin secretagogues, short term steroids and daily cyclosporine A, to combat the inflammatory nature of the disease.9 Frequent eye care visits and different treatment options impose high costs to patients and health care systems.10 Due to their non-invasive nature and low side effect profile, artificial tears have remained the main stay of therapy for DES.11 Almost all tear substitutes rapidly replace the moisture layer of tears12 and quickly reduce the symptoms. In USA, approximately 7 to 10 million Americans spend 320 million dollars per year on artificial tear products.13 In USA, many clinical trials have been conducted to evaluate their efficacy and to compare them with each other.14 In report of Dry Eye WorkShop (DEWS) was concluded that although many topical lubricants with various viscosities improve symptoms there is no evidence to suggest that any one agent is superior to another.7 However, ocular surface inflammation can be exacerbated by the presence of preservatives. Benzalkonium chloride (BAK) is a preservative frequently used in ophthalmic preparations. In patients with mild dry eye, benzalkonium chloride containing products may be well tolerated when used four to six times a day or less. Preservative free formulations are also indicated for those with a known history of allergy to preservatives and those who wear contact lenses.22 Preservative-free formulations are available in a variety of delivery systems. Many are supplied as single-dose units. These are often small tubes or plastic ampoules designed to administer one drop and to be discarded. These can usually be used to administer a drop into both eyes before discarding. In addition to the presence or absence of lipid, artificial tear formulations may be available in multidose bottles containing a preservative, or alternatively provided as preservative-free formulations in unit-dose packaging.20 Multi-dose bottle preparations of ocular lubricants are convenient to store and transport. Those licensed as medicinal products must have a 28 day expiry after opening but many ocular lubricants registered as medical devices have extended shelf lives of up to six months after first opening. Single dose units are bulky to store, particularly if several different eye drops are used. They are less convenient for the patient to carry, especially if they are being used many times a day. They have a greater unopened shelf-life than multidose vials but generate more waste.7,20 A large number of artificial tear drops are available in the market. Selecting the proper product that suits the patient, with reduced costs remains a challenge for the clinician and the patients. The present study aimed to compare the clinical efficacy of two different products of hydroxypropyl methylcellulose (HPMC) based artificial tear drops on the improvement of dry eye syndrome after four weeks.
Patients and methodsThis is a randomized patient-masked clinical trial. The subjects for this study were recruited from patients with dry eye syndrome in Tehran, Iran. At first, the subjects completed the ocular surface disease index (OSDI) questionnaire. The examination of the ocular surface and the eyelids was performed with a slit lamp biomicroscope to rule out any other ocular diseases. The inclusion criteria were: having a score >20 in OSDI questionnaire and no use of any types of artificial tears during the previous three months. The exclusion criteria were as follows: patients with an allergy, infection, or eye surface problems (e.g. pterygium); patients using contact lenses; patients using ophthalmic drugs, such as steroidal or non-steroidal anti-inflammatory, antihistamines, and glaucoma medications during the previous month, or systemically using drugs influencing tear production, such as antihistamines, cortisones, hormones, beta-blockers, antidepressants, and chemotherapy drugs; patients with a history of ophthalmic surgical operations; patients undergoing radiotherapy; patients allergic to hydroxypropyl methylcellulose; and pregnant or breastfeeding patients. For the patients who met the above criteria, the purpose of the study was explained. If they were willing to participate, they were asked to sign the informed consent form which was prepared based on the Declaration of Helsinki. The patients were examined in two visits; one before the intervention and one after 30±2 days of using the specified artificial tear. To assess patients’ DES-related symptoms, a validated OSDI questionnaire was used. The OSDI, is a 12-question survey, with a five point scale answers (0=none of time and 4=all of the time), with higher scores representing greater disability. The total OSDI score is calculated based on the following formula=100 (sum of severity for all questions answered)/4 (Total number of questions answered), where the severity was graded on a scale of: 0=none of the time, 1=some of the time, 2=half of the time, 3=most of the time, 4=all of the time. A score of 100 corresponds to complete disability while a score 0 corresponds to no disability. Following the completion of the questionnaire, the tear break up time (TBUT) assessment was performed using Lowther's method15 (A method for use of fluorescein strip in which the portion of the fluorescein strip which is designed to be wetted, is approximately 5mm wide by 15mm long to deliver a limited dose of from 0.5 to 1.0μl of liquid to the surface of the cornea) in right eye. A sterile fluorescein strip (Indicator, Tuba Teb Co., Iran) was moistened using non preserved saline and excessive solution was shaken off. The strip was touched gently to the superior bulbar conjunctiva or lid margin taking care not to instill too much solution or cause excessive reflex tearing. The patients were asked to blink two or three times naturally and then after one closure try to keep the eyes open. The time from the opening until the appearance of first dry spot was measured in seconds. The measurements were taken two times by stopwatch. For each subject, the TBUT were averaged and the average values were compared before and after the intervention. The subjects with TBUT<10s meeting the criteria for dry eye were enrolled into the study. The conjunctiva and the cornea were examined after instillation of fluorescein, with cobalt blue filter of SL-1E biomicroscope (Topcon, Japan). Punctuate staining was recorded using a standardized grading system of 0-3 for each of the five area on the corneal diagram.16 The Schirmer test was performed in right eye, with anesthetic, measuring the basic tear secretion; a drop of Tetracaine 0.5% (Anestocaine, Sina Darou, Iran) was applied to the eye. 5min after instilling one drop of tetracaine 0.5% into the conjunctival sac for test, the paper strips (Tuba Teb Co., Iran) were placed over the junction of the temporal and medial one-third of the lower eyelid margin. The eyes were closed during the test, and the length of the wet portion was measured in millimeters.17 The wetting strip counted <10mm per 5min were enrolled into the study. The subjects meeting the inclusion criteria were divided into two groups based on their score in OSDI. Artificial tears drops used into two groups were as follows: Group (A) Tear Naturale® single dose eye drops from England, Alcon company, containing dextran 70, 1mg/ml and hypromellose, 3mg/ml hydroxypropyl methylcellulose, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, zinc chloride, sodium hydrogen carbonate, carbon dioxide (to adjust pH) and purified water as non-preservative. Group B) Tearlose® multidose eye drops from Iran, Sina Darou company, containing hydroxypropyl methylcellulose 0.3g and 0.1g of dextran 70, with 0.01% benzalkonium chloride (BAK) as preservative which are available in 10mL plastic bottles with nozzle droppers and pilferproof caps. Using random blocks, the first group and the second group received Tear Naturale® and Tearlose® eye drops, respectively. The patients were blind to the names of the medication and were instructed to use the eye drops 2 times a day for 4 weeks. In addition, lid hygiene was recommended in the mornings; the base of lashes had to be cleaned at the lid margins by Eyesol Ophthalmic Cleansing Shampoo, (Livar Co, Iran). The patients were asked to return to the clinic for a follow-up visit in thirty days. Before using a statistical procedure, the preconditions for its use were verified (e.g. the normal distribution of the variables). If the preconditions were largely fulfilled, parametric methods were used for the evaluation. If the preconditions were not fulfilled, a non-parametric method was selected. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Kolmogorov–Smirnov test was performed to assess normality for continuous variables. The outcomes were compared between the two groups using an independent t-test for continuous variables or the Chi-square test for categorical variables. The comparisons of outcome measures between the baseline and 4-week after intervention in each group were performed using a paired t-test and the differences in the degree of change were compared between the two groups using an independent t-test. To compare changes in Group A and B, repeated-measures analysis of variance was used. A value of p<0.05 was considered significant.
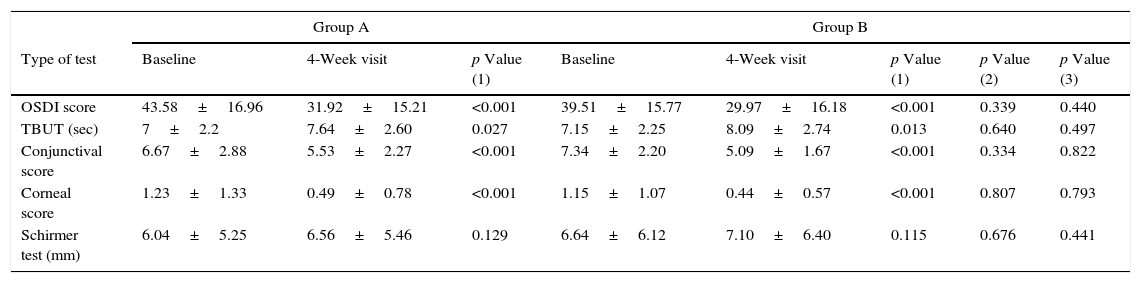
ResultsA total of 100 participants were recruited into the trial, 88 patients (41 patients in the Group A and 47 patients in the Group B) completed the entire protocol. A total of 12 subjects (9 patients in the Group A and 3 patients in the Group B) failed to complete the trial. The difference between the two groups in terms of sex distribution was not significant (p=0.135). The mean age of the participants in the Group A and the Group B was 44.08±6.29 years (range, 33–58 years) and 45.83±8.42 years (range, 31–60 years), respectively, and there was no significant difference between the groups (p=0.379). Demographic features of patients in the Group A and B are stated in Table 1. The means and standard deviations of OSDI scores, TBUT, conjunctival and corneal staining scores, Schirmer test scores of Groups A and B, before and after the intervention are presented in Table 2. The OSDI scores after the intervention showed a significant decrease compared to those before the intervention in both groups (p<0.001). However, there was no significant difference in the efficacy of the two artificial tear products (p=0.440), and repeated-measures analysis of variance did not demonstrate significant interaction between type of product and change over time in the trend of mean OSDI in the Group A and B (p=0.468). The TBUT results after the intervention showed a significant increase compared to those before the intervention, although not statistically significant (Group A; p=0.027 and Group B; p=0.013). Also, in comparing the mean results of each tests carried out, there was no significant difference in the efficacy of the two products (p=0.497). The difference of interaction between the two groups and change over time in the trend of mean TBUT was not statistically significant (p=0.834). The conjunctival staining scores 4-week after the intervention showed a significant decrease compared to those before the intervention (p<0.001). The two artificial tear products compared in two groups showed similar effects (p=0.822). Furthermore, the interaction of product type and change over time in the trend of conjunctival staining score between the Group A and B was not significant (p=0.080). The corneal staining scores after the intervention also showed a significant decrease compared to those before the intervention (p<0.001). However, two products of artificial tear drops did not show different effects (p=0.793), and the interaction the type of artificial tear and change over time in the trend of corneal staining score was not statistically significant (p=0.894). The Schirmer test scores, after the intervention showed an increase compared to those before the intervention, but the differences were not significant in either group (Group A; p=0.129 and Group B; p=0.115). Both products showed similar effects (p=0.441), and the interaction between type of artificial tear and change over time in the trend of Schirmer test was not significant (p=0.475).
Demographic features of patients.
| Group A (N=41) | Group B (N=47) | p Value | |
|---|---|---|---|
| Mean age±SD | 44.08±6.29 (range: 33–58 years) | 45.83±8.42 (range: 31–60 years) | 0.379 |
| Male/Female | 19/22 | 21/26 | 0.135 |
Group A: patients assigned to use of the product of Tear Natural® single dose eye drops.
Group B: patients assigned to use of product of Tearlose® multidose eye drops.
p value: p values were derived from an independent t-test for continuous data and the Chi-square test for categorical data.
N: number of patients.
Comparison of the clinical characteristics of the two groups.
| Group A | Group B | |||||||
|---|---|---|---|---|---|---|---|---|
| Type of test | Baseline | 4-Week visit | p Value (1) | Baseline | 4-Week visit | p Value (1) | p Value (2) | p Value (3) |
| OSDI score | 43.58±16.96 | 31.92±15.21 | <0.001 | 39.51±15.77 | 29.97±16.18 | <0.001 | 0.339 | 0.440 |
| TBUT (sec) | 7±2.2 | 7.64±2.60 | 0.027 | 7.15±2.25 | 8.09±2.74 | 0.013 | 0.640 | 0.497 |
| Conjunctival score | 6.67±2.88 | 5.53±2.27 | <0.001 | 7.34±2.20 | 5.09±1.67 | <0.001 | 0.334 | 0.822 |
| Corneal score | 1.23±1.33 | 0.49±0.78 | <0.001 | 1.15±1.07 | 0.44±0.57 | <0.001 | 0.807 | 0.793 |
| Schirmer test (mm) | 6.04±5.25 | 6.56±5.46 | 0.129 | 6.64±6.12 | 7.10±6.40 | 0.115 | 0.676 | 0.441 |
OSDI: Ocular Surface Disease Index questionnaire; TBUT: tear break up time; Group A: patients assigned to use of the product of Tear Natural® single dose eye drops; Group B: patients assigned to use of product of Tearlose® multidose eye drops.
p Value (1): indicates the significant level of paired t-test within A or B groups between baseline and four weeks after intervention.
p Value (2): indicates the significant level of independent t-test for comparing between A and B groups before intervention.
p Value (3): indicates the significant level of independent t test for comparing between A and B groups after four weeks.
In this study, hydroxypropyl methylcellulose (HPMC) as the active principle in Tear Naturale® and Tearlose® eye drops reduced the symptoms DES equally. The mean score of the OSDI questionnaire decreased significantly after the intervention in both groups. When Toda et al.18 studied the effect of 0.5% HPMC without any preservative on Sjögren and non-Sjögren dry eye patients, they observed that the symptoms were reduced in both groups. Nguyen et al.19 examined the effect of inserts containing HPMC on patients with DES and found that inserts were significantly effective in DES treatment. Lanz20 also compared preserved HPMC (GenTeal, 0.3% HPMC, with sodium perborate as preservative) vs nonpreserved HPMC (Tears Naturale, 0.3% HPMC, 0.1% dextran 70) and found no significant difference between the symptoms of two groups after the intervention. These findings agree with the result of this study. Lanz20 also found better improvement in the Schirmer test with the preserved Genteal. In this study, a slightly better improvement was observed with the preserved drop but the difference was not significant. This difference could be due different preservatives used (sodium perborate vs BAK) in two studies. In both groups, the conjunctival and corneal staining scores, after the intervention, were reduced significantly (p<0.001). In study performed by Climent,23 GenTeal Tears® with preservative, and preservative-free Tears Naturale® (Alcon Laboratories, Inc.) were compared. After 4 weeks of treatment, patients were evaluated with TBUT, Schirmer's test, and corneal staining as well as via a symptom questionnaire. A total of 37 patients completed the study. Both TBUT and Schirmer testing improved in the GenTeal group but not in the Tears Naturale® group (p=0.27). These differences in two studies could be due to the preservative used in the group GenTeal Tears and the amount of HPMC used in formulation of artificial tear drops. Subjective symptoms were not different between the two treatments. In this study, the results of Schirmer test (with anesthetic) before the intervention did not differ significantly from those after the intervention. According to previous studies performed,30–32 an increase in the volume of baseline tear was expected after the use of artificial tears, but no change was observed. The increase in volume might have happened shortly after the application of the eye drops and at the time of testing, a long time might have passed since the instillation of the drop. In this study, a slightly better improvement was observed with the preserved drop but the difference was not significant. In both groups, the conjunctival and corneal staining scores, after the intervention, were reduced significantly (p<0.001). The improvement of epithelial cells in the cornea and conjunctiva were in line with reduction of patients’ complaints. The absence of preservative does not appear to be advantageous in terms of reducing the corneal and conjuntival stains. Agents such as hydroxypropyl methylcellulose (HPMC), which decrease rose bengal staining in dry eye subjects,33 may either coat and protect the surface epithelium or help restore the protective effect of mucins. However, these results might be due to the short-term use of these eye drops. McCann et al.21 compared the efficacies of HPMC (Dacrisol unidose; Alcon, Italy), sodium hyaluronate (Lubristil 0.15% unidose, SIFI, Catania, Italy) and a new oil-in-water emulsion (Emustil unidose: SIFI) in the management of patients with DES caused by impaired lipid bilayer. They found that the symptoms and tear evaporation were reduced in the three groups, but the differences for sodium hyaluronate or the HPMC group statistically were not significant. This difference could be due to different preservatives used (sodium perborate vs BAK) in two studies. Peter et al.24 demonstrated that both unit-dose and multi-dose artificial tear drops are similar in terms of efficacy. Peter study observed statistically significant improvements in OSDI scores from baseline at 30 days. There were no clinically significant differences in performance in TBUT, corneal staining, conjunctival staining, and Schirmer's test among the artificial tear formulations. In Peter et al. study, subjects used artificial tears a median of three times a day, thus there may have been a difference between the adverse effects observed with preservative vs non-preservative formulations in subjects who used the eye drops more frequently for treatment of more severe dry eye. Some studies have emphasized the side effects of preservatives on eye surface and tears, including corneal and conjunctival tissue damage, loss of goblet cells, preventing the growth of new cells, and accelerating cell death.25–28 Benzalkonium chloride (BAK) is the most frequently used preservative in topical ophthalmic preparations, as well as in topical lubricants. Its epithelial toxic effects have been well established.11,29 The toxicity of BAK is related to its concentration, the frequency of dosing, the level or amount of tear secretion, and the severity of the ocular surface disease. In the patient with mild dry eye, BAK-preserved drops are usually well tolerated when used 4–6 times a day or less. In patients with moderate-to-severe dry eye, the potential for BAK toxicity is high, due to decreased tear secretion and decreased turnover.25,26,28,29 The incidence of most of the side effects depends on concentration of preservatives and long duration of their use. Although other preservatives have shown better safety profile than BAK,11 but we did not observe any side effects for short time about the drop containing BAK. Preservative-free formulations are absolutely necessary for patients with severe dry eye with ocular surface disease and impairment of lacrimal gland secretion, or for patients on multiple, preserved topical medications for chronic eye disease. Therefore, artificial tears without preservatives are still recommended for patients with persistent symptoms or those who require higher doses of the drops. Nonpreserved, single unit-dose tear substitutes are more costly for the manufacturer to produce, more costly for the patients to purchase, and less convenient to use than bottled artificial tear drops.22,23
ConclusionThe two artificial tears compared in this study, equally reduced the signs and symptoms of dry eye syndrome in four weeks.
FundingThis project is funded by department of ophthalmology, Bahman Hospital, Tehran, Iran.
Conflict of interestThe authors have no financial interest in the products discussed in this article.
This article is the result of a clinical research prepared by the research committee at department of ophthalmology, Bahman Hospital, Tehran, Iran.