Cognitive involvement in reading causes variations in the tonus of autonomic nerve system. The aim of this study was to examine the effect of short-term cognitive load on accommodation and pupils’ absolute values and temporal variability in test persons performing three different types of tasks.
PurposeWe aimed to show how cognitive tasks of different type and difficulty level affect accommodation and pupil behavior during a short time interval.
MethodsParticipants (n = 58; mean age 16.4 years, SD = 0.56) performed reading from a 10-inch LCD screen placed at 40 cm distance. Three different types of tasks (numerical, textual, and the Stroop task), each at three different levels of cognitive load were introduced. Participants had 90 s to complete each task. Accommodative and pupillary responses were measured with videoretinoscope Power Refractor 3 at 50 Hz.
ResultsPupil size was largest in the Stroop task (M = 5.20 mm, SD = 0.75 mm), followed by the numerical tasks (M = 5.02 mm, SD = 0.72 mm) and textual tasks (M = 4.78 mm, SD = 0.71 mm). Accommodative fluctuations – measured as accommodation SD – were largest in the textual tasks (M = 0.67 D, SD = 0.34 D), followed by the numerical tasks (M = 0.61 D, SD = 0.40 D) and the Stroop task (M = 0.52 D, SD = 0.21 D).
ConclusionsIn our experiment, short-term cognitive load was associated with altered pupillary and accommodative response to near tasks. In conflicting tasks (Stroop) or in performing continuing calculations, the pupils were larger; in tasks requiring logical reasoning, the accommodative fluctuations were greater. These effects can potentially be associated with current near-point stress and myopia growth models.
During last decades, computer and smartphone technology has grossly affected the amount of time we engage in performing near tasks and has hypothetically contributed to the global rise of myopia.1,2 Generally, near work is an important factor in myopia, but it´s role is not fully explained yet – it could be due to the altered physiology during reading and studying, but it could also be just due to the prolonged time spent in this setting. It is also not clear how different psychological (or other) processes might contribute to it.
In learning, by definition, the cognitive demand is high. The extra sympathetic arousal arising from cognitive load is proven to have the capacity of altering the optical properties of the visual system – most notably accommodation and pupils. The pupillary behavior is well explored: elevated cognitive activity consistently causes mydriasis (enlargement of the pupils),3-6 whilst the effect of cognition on accommodation is less clear – some studies found that elevated cognitive effort causes induced transient myopia (i.e. retained positive accommodation),7 whereas others revealed accommodative fatigue8 and decreased accuracy.9,10
Both pupils and accommodation vary in time; small changes are referred to as microfluctuations. Pupils’ physiological restlessness (hippus) is presumably caused by dynamic equilibrium of both autonomic nerve systems, with parasympathetic input being dominant.11 In accommodation, oscillations are caused mainly by cardio-pulmonary cycles12 but depend also on the absolute value of accommodation.13 In work ergonomics, the long-term decrease of pupil size is considered a reliable measure of fatigue,14,15 whereas the alterations of accommodation are not believed to be associated with fatigue.15 An important factor of oscillations might be the modality of the presentation of stimuli: when looking into the source of light, i.e. the back-illuminated digital screen, the accommodative system is not as accurate as when observing an object which is illuminated by secondary light source.16-18
Studies show that myopic progression is closely related to the duration and level of education,19-23 to school grades as a measure of engagement24 and to seasonal variations of near work intensity.25 Some authors found also a connection to intelligence24 and proposed that the prevalence of myopia could be linked to the stressfulness of the school system.26,27 For instance, in the developed Asian region with traditionally rigorous schooling system, myopia is increasing28-30 and has now reached highest prevalence on the planet, whereas recent studies in the Scandinavian region which anecdotally has one of the most flexible and student-centered school system show that myopia there is actually staying low, despite unfavorable environmental lighting conditions.31,32 There is the genetic component to the myopia growth as well – the COMET study found that parents of myopic children themselves were also more myopic than average and had higher educational levels than average.33 Asian race seems to be more prone to short-sightedness; Asian students reach higher myopia growth rates during their studies, regardless of whether they are educated in home- or international environment.34
Since learning and studying is inevitably associated with high cognitive activity, we wanted to examine whether cognition has a measurable effect on physiological properties of the optical part of the visual system, and if yes, is the effect different with different types of cognitive activities. Causing stress to the visual system might in the short run result in a fatigue and in the long run, hypothetically, represent a factor in myopia growth.
MethodParticipantsThe test group consisted of 56, 1st and 2nd year high school students (37 females, 19 males) of mean age 16.44 years (SD = 0.56), all of them being native Slovenians. Inclusion criteria were: best corrected visual acuity better than 20/25 in any eye (tested on LCD screen with SLOAN chart), normal color vision (tested with Ishihara plates), no other ocular abnormality (derived from participant history). Only ortophoric and minorly exophoric (up to 4 prisms of dissociated phoria, tested with Krimsky test) students were included. The study followed the tenets of the Declaration of Helsinki and was reviewed and approved by the Institutional Review Board at the Faculty of Arts, University of Ljubljana, Slovenia. A written informed consent was signed by all the included participants and their parents.
InstrumentsThe experiment was performed in a well-lit room with constant lighting. The test material was presented on a 10-inch LCD screen at 40 cm distance; the luminance of the background was kept constant. The text and numerals were displayed in 14 pt size, in four rows and centered in the lower portion of the screen, subtending 16° × 2.1° of visual angle. The setting allowed for natural viewing position – the center of the text was 20° below the horizontal plane.
A 90-second interval was allowed for silent reading in each experimental condition. After each interval, the number of elements read/processed was recorded based on the final fixation. The numerals then disappeared from the screen. Before the next experimental condition followed, participants were instructed to fixate a distant object (LCD screen with letters of 20/40 size at 5 m distance) for one minute. To reveal any sustained accommodation, a singular measurement of distant refraction was made 5 s before the task started and this was repeated 5 s after completing the task.
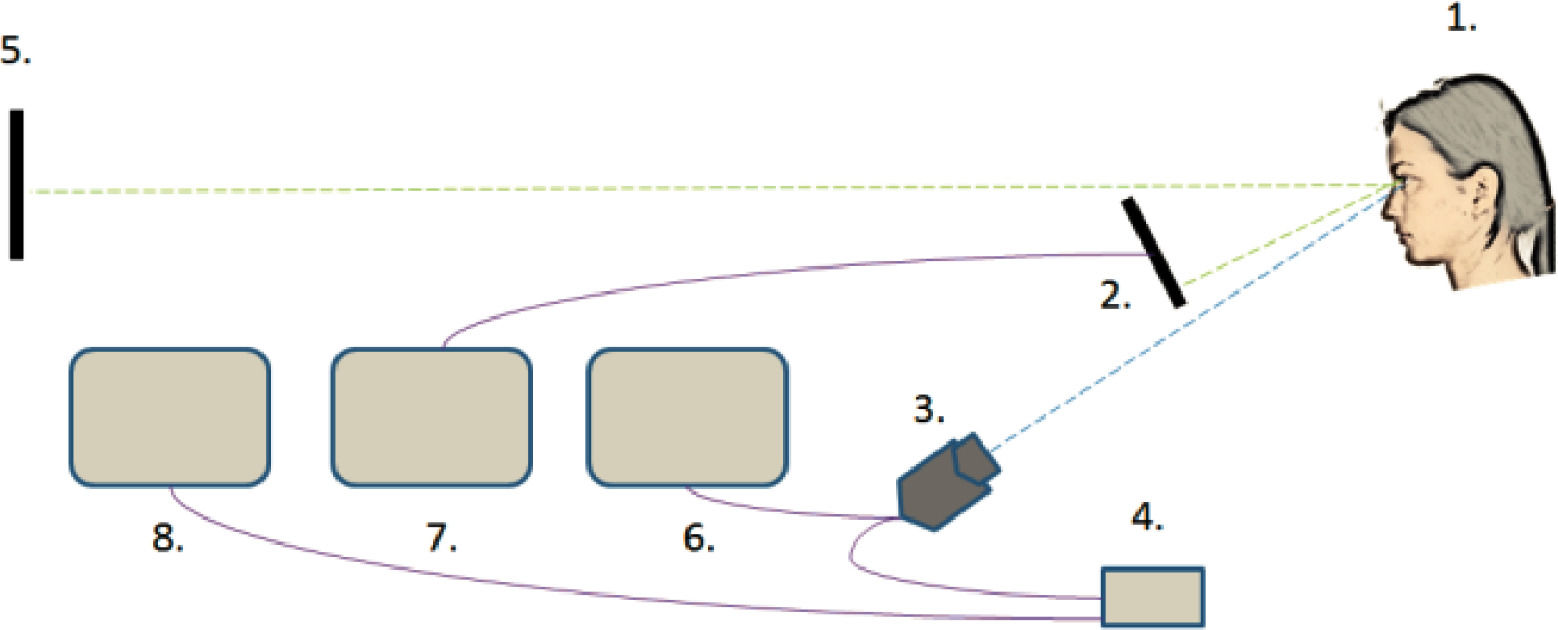
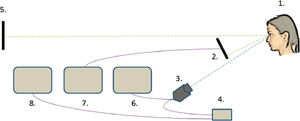
Pupillary and refractive responses obtained from the right eye were measured continuously during the experiment with Power Refractor 3 (Plusoptix GmbH, Nürnberg) in the 50 Hz mode. This instrument was proven accurate and reliable in similar studies before.35,36 The setup of the instruments is shown in Fig. 1.
The setup of the instruments: (1) The participant. (2) The LCD display. (3) The Power Refractor 3 instrument. (4) Power supply for the Power Refractor 3. (5) Distant LCD display. (6) Screen for monitoring the function of Power Refractor 3. (7) Parallel screen for monitoring the achievements of the participants. (8) Separate PC for data storage and processing.
Rather than using Power Refractor 3 in the classic positioning above the screen, it was placed below it (see Figs. 2 and 3), monitoring the pupils and refraction through a hole in a table with an average vertical eccentricity of 5,5° Thus, the measuring errors caused by blinking and closing the lids during task performance were minimized. A chin-and-head rest was used to limit participants’ movements during the experiment (Fig. 3). Their performance was tracked on a parallel screen and special observations were manually recorded.
Stimuli consisted of numerical tasks, textual tasks, and the Stroop task. Each type of tasks was presented at three levels of difficulty. The language used was the native one, Slovenian.
Numerical tasks: The basic-level task (task N0) consisted of spelling out two-digit numbers (e.g., 23, 54, 17, 33 …). Each participant was instructed to sequentially read units and pronounce them in mind. A more difficult task (task N1) required the participants to perform short calculations (e.g., 1 + 32 =) and pronounce the result in their mind, then immediately proceed to the next calculation. These calculations comprised pairs of one- and two-digit numbers where addition or subtraction of both was required, the result always being a positive number. In subtraction, there was no passing to lower decade. In the numerical task of the highest difficulty (task N2) participants performed continuous calculations, i.e. sequentially added and subtracted one-digit numbers to the result of the previous calculation, pronounced in mind the result of the calculation and then immediately continued with the next calculation in a row (e.g., 4 + 6 + 8 – 6 – 1 …). Task N0 represented minimal cognitive activity. In task N1, a small share of working memory capacity was utilized for performing the calculation. Cognitive load was greatest in task N2 which required the retrieval of information (the result of the previous calculation), transforming it (performing the calculation), and updating the information to be kept in working memory.
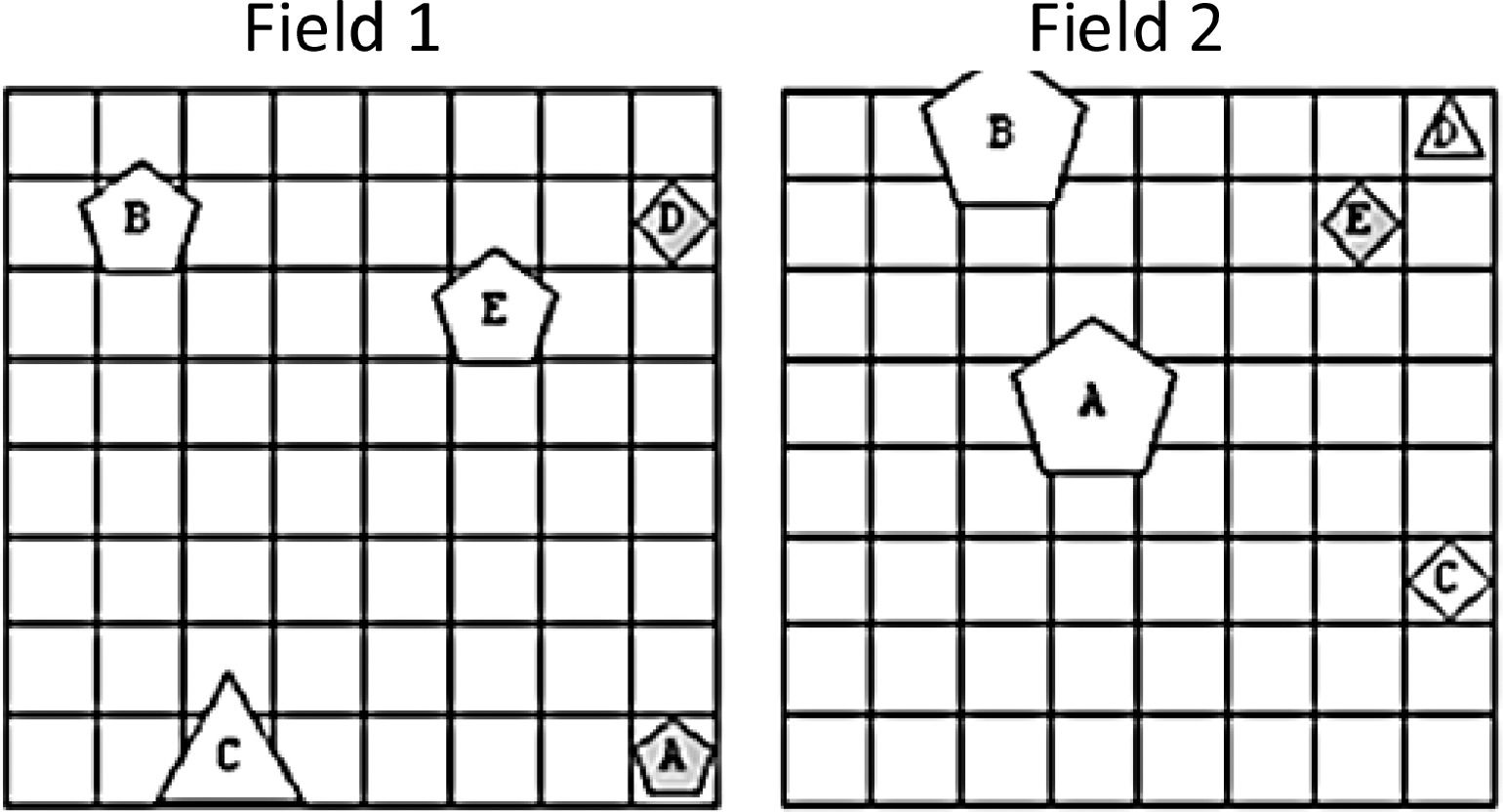
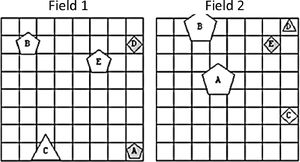
Textual tasks: The basic level task (task T0) was composed of reading a simple story (2nd year primary school level) silently and in a relaxed manner, without putting effort into remembering the content (e.g., A handkerchief fell off the girl's pocket when she was running over the field. The girl continued her way, but the handkerchief was left on the ground …). Task T1 was more difficult as it required the participants to read instructions for using an over-the-counter medication (anti-cough syrup) and try to remember the content to be able to respond to three questions afterwards (e.g., Do not take the syrup if you have severe kidney failure. After commencing the therapy, coughing should stop within 5 to 7 days …). In the highest-difficulty textual task (task T2), participants were asked to solve logical problems from an interactive internet platform Nauk.si. The task comprised statements about shapes and tints of objects drawn in two fields, as shown in Fig. 4 (e.g., The form C is a triangle and the form B is gray [Field 1: TRUE / FALSE, Field 2: TRUE / FALSE]. The form B is not a square and the form D is gray [Field 1: TRUE / FALSE, Field 2: TRUE / FALSE]) and participants had to decide whether the two-part statement was true or false for each of the two fields. In this specific task, the text was on the right to the fields and the angle of gaze subtended 16° × 7° Task T0 required minimal cognitive activity since the reading content was very simple and the participants knew they will not be asked about it afterwards. In task T1, however, the instruction was to remember the content, and the information had to be stored into long-term memory. In task T2, cognitive load was even higher due to relating textual with symbolic representations and intensive use of working memory and logical reasoning (using operations of negation, conjunction, and disjunction) during reading.
Stroop task: The classical Stroop task contains three parts. In the first part (called task S0 in our study), participants read names of colors (e.g., RED GREEN BLUE RED) written in black ink. In the second-part (task S1), they have to name the color in which sets of five letters X are printed. In the third part (task S2), color-words are displayed in a color which does not match the word meaning (e.g., the word GREEN is printed in red ink) and participants need to name the color of each word and disregard its meaning. Task S0 is the least cognitively demanding, including only reading of words which is a highly automated activity. Task S1 imposes some cognitive load since naming of colors is not an automated process. Task S2, also called the interference condition, evokes substantial cognitive load, since the automated reading must be inhibited and the color of the word must be recognized and named instead. In contrast to numerical and textual tasks, this one was performed orally.
ProcedureTasks of different types were assigned to the participants in blocks. Block order and the order of task difficulty level within blocks were randomized across participants (an example of a testing sequence would be: N0, N1, N2; S1, S2, S0; T2, T1, T0). Participants had 90 s to complete each task, each time, after completion, having 60 s to gaze at distance. The following behavioral data (task achievements) were collected: the number of pages read in the 90-second interval in tasks T0 and T1; number of statements solved correctly for both fields in task T2; number of numerals read or equations solved in tasks N0, N1, and N2; number of correct words read in task S1; number of correct names of stimulus color in tasks S1 and S2.
After completing each task, participants’ assessed their emotional response to the task and estimated cognitive load during the task by filling in a paper form. Using the Self-Assessment Manikin37 with an adjusted 9-point response scale (with the five original levels and four intermediate levels added), they assessed their emotional valence and arousal during solving the task. On numeric 9-point scales with only the extremes described verbally, they assessed task difficulty and the focus required for solving the task, with higher numbers representing higher difficulty and more focus needed, i.e. higher cognitive load.
Data analysisPupillometry and refraction data were pre-processed in MS Excel. Invalid values were removed; most prominent reasons for outliers were blinking, squeezing lids or side-drifting of gaze during mental processing. We also deleted data which were obtained at angles that were outside the measured field, data obtained when gaze direction exceeded 20° of visual angle from center, and data that were more than three standard deviations away from the mean. The control over the performance (units read or calculated) at each task was obtained by analysing saccades in the eyetracker plot. No cheating (e.g., leaving out a numeral for faster completion of the task) was noted. For each task, data on pupil size and refraction recorded within the 90-second interval were analyzed. Pupils were measured in millimeters and refraction in diopters – the refraction displayed in case of an accurate accommodative response to the stimulus in 40 cm (= 2,5 D demand) would hence be –2,5 D. Mean values and standard deviations were calculated for both parameters. The slope of the linear function used for predicting the value of the parameter based on the time passed since the start of the task was estimated using the ordinary-least-squares method.
Further analyses of subjective estimations of emotional and cognitive load were done by IBM SPSS 22 statistical package. Means, standard deviations and slopes for pupil size and refraction were analyzed by two-way repeated ordinal regressions38 in R statistical software. Statistical hypotheses were tested at the 5% alpha error rate.
ResultsTo examine whether the selected tasks truly had different difficulty levels, we analyzed test achievements and subjective estimations of emotional and cognitive load. For each task, a principal component analysis of data on emotional valence, arousal, task difficulty, and required focus was first performed to reduce the amount of data. The results of nine principal component analyses showed that a one-component solution was acceptable for different tasks (only the first component's eigenvalue exceeded 1; the component accounted for 41% to 67% of the variance of responses about different tasks). The regression method was used for calculating the component scores, one for each task. The component scores and achievements in tasks of the same type but different difficulty were then compared using a one-way repeated-measure analysis of variance. In case of violation of the sphericity assumption, Huynh-Feldt correction was used. ANOVAs and post-hoc paired comparisons (Sidák tests) showed that for all types of tasks, subjective estimations of emotional and cognitive load increased from Level 0 to Level 2 as expected, and task achievements decreased with task difficulty. Results are shown in Table 1.
Comparison of component scores reflecting emotional and cognitive load during solving the tasks and achievements in tasks of different difficulty.
| M (SD) | Results of ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|
| Task | Level 0 | Level 1 | Level 2 | F | df1 | df2 | P | Post hoc |
| Emotional and cognitive load | ||||||||
| Textual | 2.2 (1.1) | 3.5 (1.3) | 4.9 (1.7) | 77.32 | 2 | 114 | < 0.001 | 0 < 1 < 2 |
| Numerical | 3.0 (1.4) | 3.7 (1.5) | 5.1 (1.6) | 97.54 | 1.65 | 95.09 | < 0.001 | 0 < 1 < 2 |
| Stroop | 2.8 (1.3) | 4.3 (1.5) | 5.9 (1.7) | 126.84 | 1.73 | 96.79 | < 0.001 | 0 < 1 < 2 |
| Task achievements | ||||||||
| Textual | 3.2 (0.8) | 2.8 (1.0) | 14.6 (4.3)a | 417.51 | 1.08 | 61.55 | < 0.001 | 0 > 1a |
| Numerical | 136.5 (51.3) | 51.3 (18.2) | 41.9 (9.4) | 474.86 | 1.38 | 78.47 | < 0.001 | 0 > 1 > 2 |
| Stroop | 180.5 (37.2) | 127.8 (19.6) | 89.0 (17.4) | 255.58 | 1.34 | 76.12 | < 0.001 | 0 > 1 > 2 |
Note. Post hoc refers to the results of the post hoc paired comparisons with Sidák test.
Next, we examined the values of visual system settings during different tasks. Average pupil size across tasks was 4.98 mm (SD = 0.77 mm). Average accommodative response was –1.69 D (SD = 1.24 D), representing a lag of accommodation of 0.81 D.
Descriptive statistics for physiological responses to different tasks are shown in Figs. 5–7. Since many distributions of the pairwise differences between the studied parameters found in different experimental conditions were statistically significantly different from normal (Shapiro-Wilk's test P < .05), we used two-way repeated ordinal regressions38 to analyze the effects of task type and difficulty level on the studied parameters. Inside the regression, medians (Mdn) were used to address non-normality.
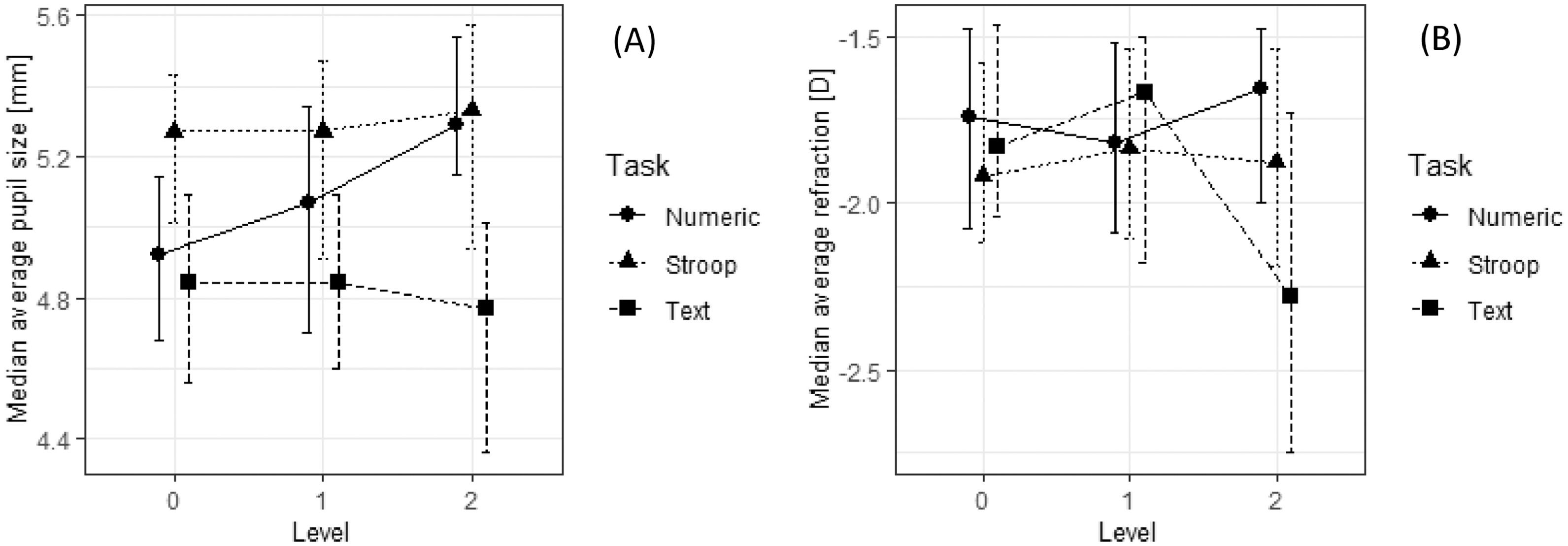
Comparison of median values for pupil size (A) and relative refraction (B) in nine tasks defined by task type (numerical, textual, and Stroop) and level of difficulty (0, 1, 2). Whiskers represent 95% confidence intervals for the medians estimated by non-parametric bootstrap with 5000 samples and BCa correction.
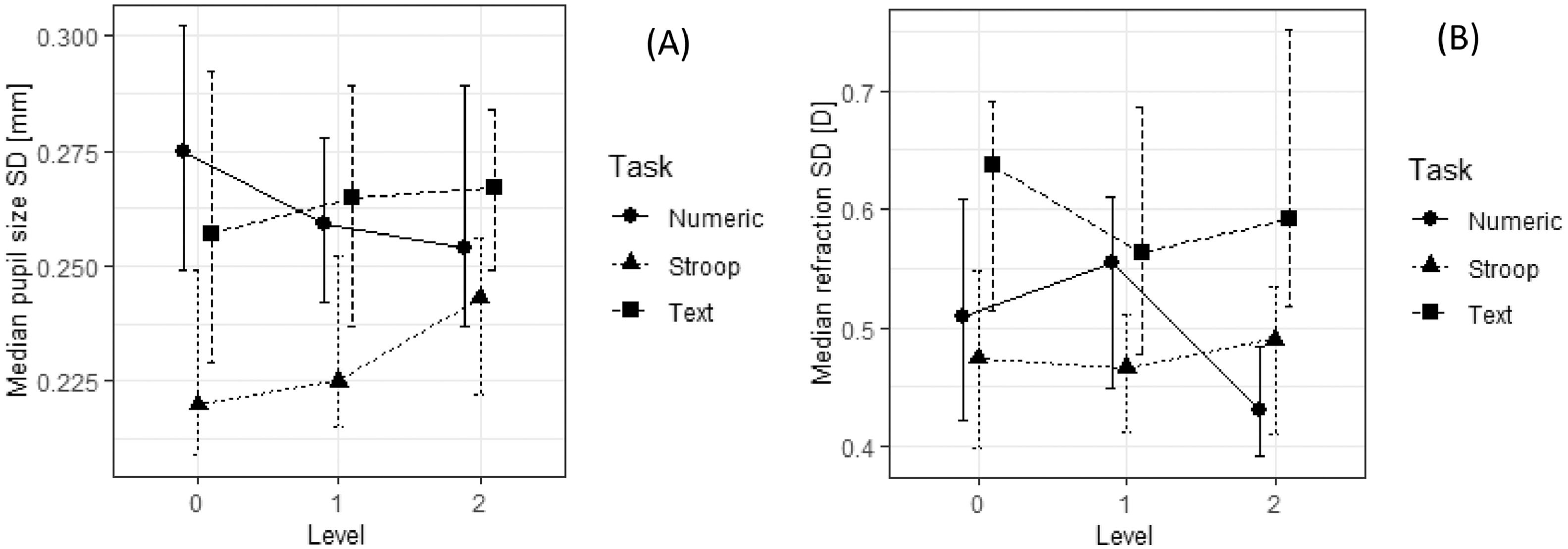
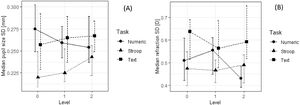
Comparison of standard deviations for pupil size (A) and relative refraction (B) in nine tasks. See also notes to Fig. 5.
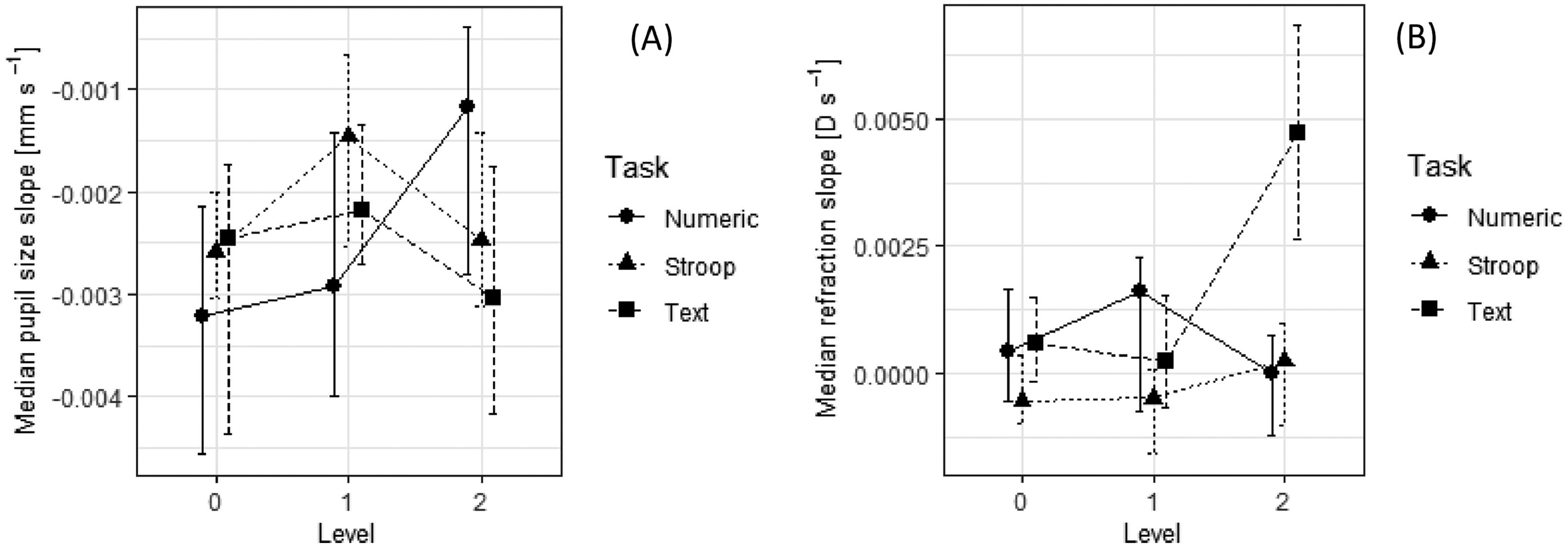
Comparison of slope parameters for pupil size (A) and relative refraction (B) in nine tasks. See also notes to Fig. 5.
A 3 (task type) × 3 (task difficulty) repeated-measure ordinal regression was used to test the effect of task type and difficulty on six examined physiological parameters. Post-hoc Tukey-adjusted comparisons were done to compare different conditions pairwise. Table 2 shows the results of these analyses. Pupil sizes were statistically significantly different in different types of tasks. Pupil size was largest in the Stroop task (Mdn = 5.30 mm), followed by the numerical tasks (Mdn = 5.11 mm) and textual tasks (Mdn = 4.79 mm). Different types of tasks also resulted in different pupil standard deviations––in the Stroop task (Mdn = 0.23 mm) pupil standard deviation was slightly lower than in the numerical and textual tasks (Mdn = 0.26 mm). Tasks also differed in all three refraction parameters: in the average accommodation (its median value for the textual and Stroop tasks was –1.88 D and for the numerical tasks it was –1.74 D), in accommodation standard deviation (median SD was 0.47 D for the numerical tasks, 0.48 D for the Stroop tasks, and 0.58 D for the textual tasks), and in accommodation regression slope (median slope was 0.0005 D s − 1 for the numerical tasks, –0.0004 D s − 1 for the Stroop tasks, and 0.0013 D s − 1 for the textual tasks).
Results of two-way repeated ordinal regressions for different physiological parameters.
Note. R2 = Nagelkerke R2.
Task difficulty affected mean pupil size in the numerical tasks but had no such effect in the textual and Stroop tasks (the interaction term was statistically significant; see Table 2). The decrease in pupil size over time was smaller in the difficult numerical tasks compared to the easier ones, whereas in the textual and Stroop tasks no systematic effect of task difficulty on the slope parameter could be observed. To sum up, parameters of pupil size seemed to change systematically with task difficulty only in the case of the numerical tasks.
For the accommodative response (refraction) parameters, the interaction between task difficulty and task type was statistically significant, but no systematic and interpretable effects could be noted. We can see in Fig. 5B that task T2 which required intensive logical reasoning resulted in the smallest average lag of accommodation among all experimental conditions (average refraction value was closest to –2.5 D). However, at the same time the largest increase in the lag of accommodation with time was observed in this task (see Fig. 7B; the total change in refraction – increase in lag of accommodation – was approx. 0.43 D in 90 s). Even though the accommodative response in S2 condition was not statistically significantly different from the one measured in S0 and S1 condition, we noticed that task S2 evoked a lead of accommodation (excessive accommodation for the distance given) in 15 (27%) out of 56 participants. The effect, however, was not associated with any other measure.
Inspection of the correlations between different physiological parameters within tasks showed that in many tasks (but not all) the standard deviation and slope parameters were correlated. This might indicate that the change in pupil size or refraction with time was relatively consistent (toward larger or toward lower values). Achievements in different tasks were not correlated to accommodative or pupillary changes. The correlations of the component scores and the examined physiological parameters in various tasks were also very low (the correlation value that deviated most from 0 was –0.32).
The analysis of retained accommodation revealed no significant change of refractive posture; in the pre-test stage it was on average +0.045 D (SD = 0.42 D) and in the post-test stage it was on average +0.111 D (SD = 0.46 D), t(55) = –1.84, P = .121. There was also no statistically significant difference between emmetropes (n = 46) and corrected myopes (n = 10). In emmetropes, the pre-test refraction was +0.016 D (SD = 0.45 D) and the post-test refraction was +0.114 D (SD = 0.49 D), t(45) = –2.25, P = .057. In corrected myopes, the (relative) refraction in the pre-test measurement was +0.175 D (SD = 0.29 D) and in the post-test measurement it was +0.100 D (SD = 0.24 D), t(9) = 2.00, P = .081. It is to note that the change, although not statistically significant, was towards plus in emmetropes and towards minus in corrected myopes.
DiscussionThe study has shown that there is a general effect of cognitive processing on accommodation and pupillary response. The effect of cognitive activity on pupils has been explored before and it is now postulated that it takes place at the level of oculomotor nucleus.39 Our findings show that accommodation, too, is influenced by cognition, supporting the hypothesis about the locus of the effect, since both accommodation and pupil size are regulated from this same part of the brain.
It was previously reported that non-congruent tasks (i.e. tasks not related to the target observed), evoked less accurate accommodative response.40 In our study, however, different types of cognitive load were employed. Data processing in reading words and numbers and naming colors follows different paths.41 The fMRI technology revealed that the left parietal cortex is involved in the coding of the position of letters and their identity and it thus contributes to the early part of the process of reading.42,43 Its role might be in controlling the robustness of processing information. Carreiras et al.31 have shown that the robustness for changes within a set of characters is higher for letters than for numbers, which makes sense, since the rationale for reading is understanding of the whole words (and sentences, paragraphs, etc.). In numbers, on the other hand, the accurate information is defined by non-interchangeable order of numeric characters, so, contrary to the robustness in letters, high processing accuracy is required. In the Stroop task the situation is different again: in the interference condition where the naming of text color is required, the visual system must use the color channel only and resist the temptation to read the text, so a high level of robustness of letter processing is needed. For good performance in the Stroop task, high flexibility of choosing the correct processing channel in different conditions and strong inhibition of irrelevant visual information is important. The three different types of tasks in our study evoked essentially different ocular responses. It is interesting that the T2 task requiring logical reasoning evoked more accurate accommodation (smaller lag of accommodation) and smaller pupils than the S2 and N2 tasks, which is in favor of the robustness of text processing hypothesis, however, there were more oscillations of the refractive value in this condition. It is also interesting to note that in the Stroop interference task (S2) and the continuous calculations task (N2), the changes in the visual parameters across task difficulty were most congruent of all measures.
Pupils were significantly larger when performing the Stroop task than in performing the textual tasks. The reason for this might be the presence of incongruent stimuli in task S2 and the use of color naming in task S1 which increases cognitive load. However, compared to the T0, T1 and T2 tasks, pupils were also larger in the S0 task containing no incongruent stimuli, only reading of color words. The S0 task could therefore be regarded as a textual task as well. The difference between the textual tasks T0, T1 and T2 and the S0 task is that the first ones always included sentences containing a subject and predicate and some redundancies. In such sentences, some information may be removed but the meaning is still uniquely defined; the redundant information makes the text more readable. In contrast, the S0 task only included adjectives with no redundancies present, so the text was less readable, thus presumably requiring more attention for processing. Our results could lead to the conclusion that reading the content with little redundancies or the text which we do not understand or has no meaning for us affects pupil diameter.44 The increased pupil size is not in favor of physiology in reading, as the depth of focus decreases and allows the aberrations of higher order to worsen the image quality. At the same time, low readability, poor understanding of the text, or making lots of effort to understand the text could hypothetically be risk factors for myopia.
Many studies exploring cognition in near work used numbers or calculations to vary the cognitive effort.4,7,40 With the selected numerical tasks we found effects on pupils and accommodation that were different from the effects of other tasks – the pupillary oscillations in the numerical tasks decreased with increasing difficulty, whereas in the textual tasks the trend was opposite. Similarly, the accommodative oscillations decreased with highest cognitive load in the numerical tasks, and an opposite tendency was found in the textual tasks. With this in mind, it is interesting to note that myopia growth was found to be greater in students who were more linguistics oriented than in those who had better mathematical skills.24 In accordance with Langaas et al.36 and Harb et al.,45 we assume that, just like larger pupils mentioned before, also the oscillating refraction might be a factor associated with the axial elongation of the eye due to higher cognitive demand during reading. According to the results of our study, this association may be more pronounced in reading than in performing mathematical tasks.
The experiment, using short duration of experimental tasks (90 s), revealed no significant retained accommodation, neither in emmetropes, nor in myopes. However, the tendency of myopes shifting towards short-sightedness was noted, which is in accordance with other research findings that found a shift in tonic accommodation due to near work in young myopes.46
Limitations of the studyThere was a large inter-individual variability in the studied physiological parameters and participants’ responses to different tasks. Even though similar studies4,7 typically presented the same tasks to all the participants, cognitive abilities of different participants may vary a lot, which is why a certain task might have a different difficulty for different participants. Cognitive abilities of the participants should therefore be controlled in future studies. A large pool of tasks of varying difficulty could be prepared in advance and tasks of comparable difficulty levels could be chosen individually according to each participant's ability (e.g., item-response theory could be applied for choosing tasks of comparable difficulty for different participants).
Future studies should use stimuli that occupy an even smaller space than in our study to minimize the effects of different patterns of gaze shifts that might influence the measurements. A running infinite line of text or numbers could be useful for this purpose. Ideally, stimuli that elicit similar saccades should be used, and an eye-tracker should be used to monitor eye movements.
The 90 s interval for a single task is a rather short one and might not allow the change in the sympathetic system to manifest fully. Hence, studies with longer periods of tasks should be conducted in the future.
ConclusionIn the study, we found important influences of cognitive effort on pupils and accommodation. We discovered that pupil size was a good measure of cognitive effort in numerical tasks. However, in other domains, such as textual tasks or the Stroop task, pupils did not reliably represent increased mental activity. Accommodative responses varied more in textual tasks than in tasks in the other two domains. In the most difficult condition with textual information that required logical reasoning, the accommodative response decreased significantly over time (the accommodation lag increased). In the numerical task with the highest level of difficulty, the oscillations of accommodation were the smallest. Thus, the results of our study show that the effects of cognitive load vary across domains and are therefore not generalizable. Although our results are not fully conclusive, we can hypothesize that when performing demanding near tasks, at least one physiological parameter is altered toward less favorable values. Pupils are larger in conflicting tasks and when reading texts with low readability (as in the Stroop task) or when performing continuous calculations and the change in accommodative response over time is larger in tasks requiring logical reasoning and inference. These findings are consistent with current views on the effects of education on myopia growth and point to some potential risk factors.
In clinical practice, good myopia management includes optical and pharmacological interventions, but also advising about environmental factors. Patients are counseled on how to perform near work and how to adopt good ergonomics. We hypothesize that when reading, the content presented to young progressing myopes should be well matched to their comprehension ability, as excessive cognitive load appears to be a disturbing factor in the physiology of near vision. In addition, we would encourage school systems to favor clear, readable, and comprehensible texts and to encourage students to releasing strain while reasoning, if possible, for example, by fixating distant objects. Alternative ways of conducting high cognitive load learning activities, such as outdoor content discussions, might work as preventive strategies. Further studies should be conducted on the optical properties of the visual system in different learning situations to better understand potential educational risk factors for myopia growth.