Personal mobile devices such as smartphones are proving their usefulness in ever more applications in tele-eyecare. An inconvenience and potential source of error in these past approaches stemmed from the requirement for the subjects to situate their devices at a distance. The present study aims to clinically validate best corrected visual acuity (BCVA) measures carried out by a novel smartphone application “vision.app” (VisionApp Solutions S.L.) using comparative statistics against clinical measurements.
Materials and methodsBCVA was measured in both eyes of 40 subjects using vision.app which displayed a black Landolt-C optotype with crowding on a white background, and utilized a 4 forced-choice procedure for the subjects to find (by means of swiping in either of four directions) the smallest optotype size they could resolve. Results were compared to BCVA measurements taken using a standard Snellen chart placed at 20 feet (6 m).
ResultsThe t-test revealed no significant differences between the app- and clinically-measured VA (p = 0.478 (OD) and 0.608 (OS)), with a mean difference between clinical and app measurements of less than one line of the eye chart (-0.009 logMAR (OD) and -0.005 logMAR (OS)). A limit of agreement for a 95% confidence interval of ± 0.08 logMAR for OD and OS was found.
ConclusionsThe results show the potential use of a smartphone to measure BCVA at a handheld distance. The newly validated study results can hold major future advancements in tele-eyecare and provide eye care professionals with a reliable and accessible method to measure BCVA.
In the wake of the COVID-19 pandemic, there have been rapid policy changes to expand the utilization of telemedicine across all medical specialties. In 2018, certain eye health organizations, such as the American Academy of Ophthalmology (AAO) predicted a sharp rise in the utilization of teleophthalmology in the coming years.1 When this rise in utilization came sooner than expected, the AAO urged ophthalmologists across the USA to reduce non-emergent office visits to decrease transmission between providers and patients.1
A major driving force behind the recent emergence of teleophthalmology is the immense and expanding market of smartphones and tablets together with their high performance as computing and sensing devices. In the last decade the advances in the organic light-emitting diode (OLED) screen technology have permitted these devices to attain pixel densities suitable for clinical utility in teleophthalmology applications. Most smartphones can generate screen resolution up to 4 K (more than 8 M pixels) with luminance values larger than 1000 nit and pixels capable of generating 90% of the colors visible to the human eye. High-speed microprocessors allow these devices to display and analyze HD videos at speeds exceeding 30 Hz.
Visual acuity (VA) is a visual quality parameter used to describe the spatial resolving power of a visual system. It is related to the smallest angle subtended by an object that can be identified by the observer.2 Deficits in VA may be localized. Visual acuity may be compromised by optical pathologies of the cornea or crystalline lens, or can be attributed to anatomical defects of the deeper structures including those of the retina in pathologies such as diabetic retinopathy and macular degeneration.3 Front-facing cameras can be utilized by some apps to determine distance from the device to the subject in real time.4–6
In clinical practice, the term “visual acuity” usually corresponds to the best corrected visual acuity (BCVA), which is defined as measured with sphero-cylindrical correction (glasses or contact lenses). In practical terms, BCVA mainly reflects the neural stage of visual processing, since these measurements are obtained when the patient wears lenses with their best correction, bypassing the potential optical problems generated in the optics. There are few optical causes which can reduce the retinal image quality of a corrected eye such as the presence of abnormal intraocular scattering due to cataract7 or high-order monochromatic aberrations. On the other hand, chromatic aberration does not seem to play an important role in VA.8 Examples of pathologies that can give rise to large values of aberrations that can affect BCVA are: keratoconus9,10 or severe corneal modification such as in the case of penetrating keratoplasty.11–13
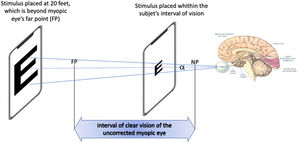
Visual acuity depends on parameters of the viewed object such as contrast, color or luminance of the background.14 Standards of measuring it such the British Standard BS 427,414 have been developed. It is also affected by the distance to the object in non-corrected eyes, (e.g. in myopic eyes). However, when measurements are made within the range of clear vision (i.e. close to the eye in case of myopia), VA should be correctly measured considering only angular size, i.e. for a particular VA, as the stimulus approaches, the font size must decrease accordingly to keep the angular size constant (Fig. 1). This assumption is based on the fact that VA is practically not influenced by accommodation, as described in detail in the Discussion section. Then, BCVA could, in theory, be measured using appropriate stimuli placed within the subject's interval of clear vision.
Measuring BCVA in an uncorrected myopic eye by situating the mobile device within the interval of clear vision. When the smartphone containing the stimulus is placed between the far point (FP) and the near point (NP), and with the angular side of the stimulus kept constant, BCVA can be found by finding the angle α of the smaller readable letter.
There are numerous methods of measuring visual acuity, although most mobile applications (apps) are published without external testing for validity or reliability before being used in clinical practice.15,16 According to a study that reviewed 35 apps designed for remote visual acuity measurement, none were suitable for virtual consultation.16 Reviewers determined the apps lacked sufficient accuracy or patient accessibility for clinical use. Additionally, symbols or letters used by the applications were not consistent among patient and provider devices.17,18 Most applications use optotypes that remain a static size, which forces the subject to conduct the test at a specified distance.15,17,18 Not only does potentially compromise the integrity of the measurements, but also requires the use of a tape measure or similar device, which may not be available in remote areas.18 Some applications rely on the recognition of a symbol such as a letter which may not be feasible for certain populations, such as pediatric patients or those with varying levels of literacy.19
While telemedicine has always been an attractive means of serving patients remotely, the COVID-19 pandemic highlighted the critical need for an accurate, user-friendly teleophthalmology platform. The present study aims to address this need through the clinical validation of BCVA measured using a mobile application developed to be used handheld and to be on par with the clinical BCVA, assumed to be the gold standard.
Material and methodsParticipants40 participants (n = 80 eyes) were recruited through email correspondence. Age of the participants ranged between 21 and 71 with a variable history of myopia and need for correction. Clinically measured best-corrected visual acuity (BCVA) ranged between 1.50 and 0.67 decimal (−0.18 and 0.18 logMAR). The measurements we obtained independently in two institutions: University of California, Irvine (UCI) and Universidad de Murcia, Spain (UMU), being the number of subjects in UCI and UMU of 18 and 22 respectively. Institutional Review Board Committee approvals were obtained in both institutions. The study was conducted in compliance with the United States and European Health Insurance Portability and Accountability Act (HIPAA) and adhered to the tenets of the Declaration of Helsinki. Following the recruitment of study subjects, each participant was provided with a patient characteristic data collection form. Patient gender, age, history of myopia, family history of myopia, habitual lens power, clinical ocular history was collected prior to clinical measurement.
App & devicesEach form was associated with an ID identifying the patient and device used for vision.app measurement to be utilized for later data analysis. Clinical measurement of VA was performed by an ophthalmologist or optometrist prior to the app measurements to establish a clinical baseline to which the smartphone measurements would be compared. BCVA measurements were taken for each eye using a standard Snellen chart placed at 20 feet (6 m). The room illuminance was the same in clinical and app measurements as both were taken with the subjects in the same place. Clinical prescription lens parameters including sphere, cylinder, and axis were collected during the clinical evaluation using a lensometer.
5 different devices were utilized for the measurement: Samsung Galaxy Tab S2, Google Pixel 3, Xiaomi Redmi 9C, Huawei Mate 20 Pro and a Huawei P20 Lite. Specific device characteristics are detailed in the table below (Table 1).
Testing procedureA novel mobile app, myopia.app© (VisionApp Solutions S.L., Spain), was installed in the devices which used the Android OS (Google). A previous version of the app has been used on previous studies to measure the face distance20 and the face illumination.21 The app includes several features, one of which is the measure of BCVA. The app tests the user's ability to recognize the orientation of a Landolt C optotype to simulate measurements of visual acuity in a clinical setting. The app configures the brightness of the mobile display to the maximum automatically each time an AV test is going to be carried out, thus ensuring background luminances larger than 300 nit and Michelson contrast higher than 95%. With the use of the front camera of the device which allows us to measure the face-device distance, the optotype size shown on the screen is scaled to the desired stimulus visual acuity. At the end of the assessment the app reports a measure of VA to the eye care professional.
As a preliminary step, the subject was asked to remove their masks and hold the device at a calibration distance of 30 cm from their face and confirm. The subject was then prompted by the app to follow the instructions to measure visual acuity at a comfortable hand-held distance.
The app displayed a black crowded Landolt C optotype on a white background and used a 4-force choice procedure based on FrACT (Freiburg Visual Acuity & Contrast Test).22 The algorithm uses a best PEST (best Parameter Estimation by Sequential Testing) procedure in which a psychometric function having a constant slope on a logarithmic acuity scale is assumed. 24 letters are shown including two “bonus” letters that the subject can clearly see to build confidence that the task has been done correctly. The algorithm initially displays a low AV optotype (0.9 logMAR). A limit of 1.5 decimal VA (−0.18 logMAR) is imposed to avoid the limitations of the screen to show very high spatial frequencies. The screen resolution is indeed an important limitation when the dpi of the phone is not too high and the test is performed at a relatively close distance. We overcame this limitation by forcing the user to do the test at a minimum distance which was calculated internally by the app taking into account the dpi of the screen and a maximum measurable decimal VA of 1.5 (spatial frequency of 45 c/g). When the subject placed the phone at a distance lower than the minimum distance, the screen displayed a warning to the subject to move away from the screen, the warning disappeared when the actual distance was equal or larger than the minimum distance. The subject swiped their finger in the direction of the opening in a Landolt ring. If the patient was unable to identify the opening, they were given the option of selecting a button at the bottom of the screen (labelled “I don't see it”) indicating that they were unable to resolve the stimulus. If the subject could not identify the letter for a third time, they were asked to guess. Following measurement of VA, the app displayed a calculated visual acuity in decimal format, which was recorded.
RepeatabilityBCVA was measured four times per eye at a hand-held distance of approximately 30 cm using a smartphone. The same device was used to perform 4 consecutive BCVA measurements in the right eye and later, using the same device under the same conditions, 4 measurements were performed in the left eye.
Statistical analysisThe agreement of measurement methods was assessed via a Bland-Altman statistical analysis based on a two-tailed paired t-test or a Wilcoxon Man Whitney in case the assumptions of normality and homoscedasticity are not fulfilled. These statistical methods were selected since we can assume normality and homoscedasticity of the sample after performing the Shapiro Wilk and F test following the Levene's criterion. The mean difference between the measured VA of vision.app and the traditional measurement, t-test statistic, associated standard deviation, and power of BCVA was calculated. All recorded decimal measures were converted to logMAR format to conduct the statistical analysis. Following the statistical method of C. McAlinden,23 an ANOVA test was performed for repeated measures in order to evaluate the existence of statistically significant differences between measurements in the same eye. RStudio v. 2022.7.2.576 was the program used for the statistical analysis.
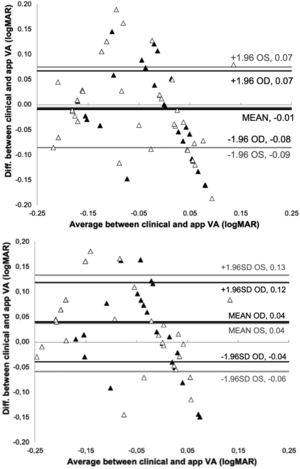
ResultsFig. 2 show the Bland-Altman plot of the comparison between the VA measured clinically and the app after 4 and 2 repeated measurements.
With 4-repeated measurements the assumptions of normality and homoscedasticity are fulfilled after performing the Shapiro Wilk test obtaining a result p = 0.525 (OD) and p = 0.417 (OS) and a F test following the Levene's criterion, to compare the two variances obtaining a result p = 0.141(OD) and p = 0.256 (OS). In addition, the t-test revealed no significant difference in measured VA between vision.app and clinical measurements (p = 0.478 and p = 0.608 for OD and OS, respectively). The mean difference between BCVA measurements in logMAR was - 0.009 ± 0.039 and - 0.005 ± 0.041 for OD and OS, respectively. Agreement between VA measurements taken by vision.app are consistent. The repeatability analysis shows statistically significant differences between measurements of the right eye (p = 0.018), while there are no statistically significant differences for the left eye (p = 0.102).
With 2-repeated measurements the assumptions of normality and homoscedasticity are not fulfilled so non-parametric Wilcoxon Man Whitney text obtaining non statistically significant differences between the clinical and app measurements for both eyes (p = 0.865 (OD) and p = 0.653 (OS)).
Given the relative low sample of 40 subjects and the SD values obtained a statistical analysis was carried out using an open software (G Power 3)24 to find the power of the statistic used for a different between the clinical and app values. For a significance of p = 0.05 and considering a clinically significant difference of 0.1 logMAR, the statistical power obtained from the difference between both methods is 0.95.
DiscussionA mobile application to obtain the BCVA at a handheld distance has been investigated for the first time. The app aims to effectively address the shortcomings of its predecessors in the areas of accuracy of measurement, consistency, operating distance, and patient accessibility. As previously mentioned, the app uses a Landolt C optotype and adjusts the letter in size according to the face-device distance. This facilitates self-measurement at a handheld distance, making it more attractive for use by individuals with mobility limitations or those who live independently or in low-resource areas. It also improves the accuracy of VA measurements, as subjects will no longer inadvertently be too close or too far from the device. Instead of asking the subject to identify a symbol (“A” vs “B”), the app employs a much simpler recognition of “open vs closed” on the Landolt ring which improves accessibility across different languages and literacy levels.
The t-test revealed no significant differences in measured VA (p = 0.478 and p = 0.608 for OD and OS, respectively), with a mean difference between clinical and app measurements of less than one line (−0.01 logMAR). Fig. 2 shows that the mean difference between measurement methods was −0.01 logMAR (95% CI), with a limit of agreement of ± 0.07 logMAR, for 4 repeated measurements in both eyes. The results in Fig. 2 also indicate that 2 repeated measurements are needed to get a limit of agreement of ± 0.08 (OD) and ± 0.1 (OS) logMAR (corresponding to the limit of clinical significance). This means that only 2 measurements of VA with the app are enough in normal conditions. In case that only one VA measurement is taken with the app we have found that the limit of agreement would be ±0.15 logMAR, similar to the value found clinically25 and slightly lower than the results found by M. Back for test-retest reproducibility band using the FrACT (± 0.2 logMAR) .26
Several participants complained of dry eye while testing. The discomfort may be a potential source of error in the study as VA may have been progressively compromised due to subject fatigue or tear film disruption. It may be prudent to consider providing eye drops to study participants or reduce the number of trials performed in future studies. Assuming a clinical minimum difference of one line (0.1 log MAR), we have found 86% of subjects below a clinical difference between both methods taking into account that 4 repeated measurements were taken, while clinically it is usually only taken once.
We have found no significant differences between the results of the difference between clinical and app, obtained by the two research centers collaborating in the study (p = 0.32 (OD) and p = 0.61 (OS)).
The results indicate that BCVA can be measured when the object (in this case a Landolt C) displayed on the smartphone or tablet screen is placed closer to the subject within their interval of clear vision. This is expected since VA is a measure of the eye's resolution, related to the minimum angular size of the object rather than the actual distance. Indeed, several studies show that VA does not change with accommodation if the object is properly scaled.27,28 This result can be explained from a physiological optics point of view considering that the size of the retinal image accommodated changes very little (about 1% for 7 D of accommodation) than that of a relaxed eye.29
Moreover, the optics of the eye do not change very much during accommodation. For instance, chromatic aberrations almost do not change with accommodation30 and the largest monochromatic aberration that changes during accommodation is defocus (i.e., lag increases with target vergence,31–33 which may cause a large lost in the retinal image quality. However, lag is very depending on the side of the stimulus.
A series of studies of the accommodation response made by Heath in 1956 clearly showed that the larger the letter size, the larger the lag of accommodation, so the slope accommodation response decrease well below 1 (ideal response).28 In this regard, it has been recently shown that accommodation response keeps nearly one (no lag) while the subject tries to perform a measurement of his VA, and very small letters are used as stimulus.34 Thus, a proper rescaling the stimulus (see Fig. 1) will reduce the potential accommodation error.
In regards with astigmatism, it is also known that does not practically change during accommodation.35–39 A potential source of discrepancy between the measurement of BCVA in placing the stimulus within the interval of clear vision at 20 feet from the corrected eye versus the uncorrected eye is when vision is compromised by a large astigmatism. In this case, the interval of clear vision will correspond to the intersection between the rays of light arriving in the eye parallel to the axis of the astigmatism and another corresponding to rays of light perpendicular to the axis. If these two intervals do not intersect it could represent a limitation of the methodology used. However, in practice, there is not such a limitation for two reasons. Firstly, subjects that does not have any interval of clear vision in each eye are usually presbyopic subjects with relatively large astigmatism and they normally use progressive lenses so they can perform the test with the smartphone while wearing their correction which includes their addition. Secondly, if the smartphone shows a Landolt C with the aperture in a certain direction, the eye will unconsciously accommodate to maximize the retinal image quality and detect the aperture in case the letter is large enough.
Finally, regarding high-order aberrations, it is also well known that they do not change dramatically during accommodation.40 The main changes are due to the decrease of the spherical aberration, usually passing from a positive to a negative value,41 with minimal influence on the measured VA.41
The promising results of this study that show a high agreement between app and clinical BCVA have major implications for the field of ophthalmology and optometry as the need for effective telemedicine becomes more apparent. Our results suggest that a reliable measurement of BCVA can be obtained using a smartphone with an app able to rescale the size of the stimulus accordingly to the distance between the device and the subject and placing the device within the interval of clear vision of the naked eye. Different types of tests can be used, although the use of the Landolt C or Snellen E optotypes increases the app's utility for diverse populations with varying degrees of literacy.
While the results of this study are encouraging, there are limitations and issues that must be acknowledged. First, the study population itself was relatively small (n = 40) with a wide range of baseline BCVAs. In future studies, it would be ideal to test a larger population, as this would improve the overall external validity. Another two limitations are intrinsic to the methodology itself. Firstly, there is an assumption that the smartphone can be placed within the interval of vision of the naked eye, but that interval can be placed far away as is the case of emmetropic presbyopics, or even, outside of a real interval (as is the case of a hypermetropic presbyope). Secondly, as mentioned in the Introduction, eyes with large high-order aberrations or scattering (cataract) can have optical defects that cannot be corrected by placing the smartphone in the interval of clear vision, since such an interval does not exist. It can be argued that the same would happen in the corrected eye by means of sphere-cylindrical lenses, so the methodology could be applied. However, it is well known than a decrease in the retinal image quality (i.e., scattering) may reduce the accommodation response.28,42 This adds additional deterioration (caused by the lag) in the case of BCVA measured at a middle point of the interval of vision versus when it is corrected at a far point, as is the case of the standard clinical refraction.
Moving forward, the results of this study may be used as a foundation for other studies utilizing a similar methodology in specific situations or for certain targeted populations. One possible avenue is to validate the app used in this study in a pediatric population in which some patients may have difficulty focusing on the traditional Snellen chart placed at a further distance. The app could provide a convenient and quick way for parents to be involved in the eye care of their child.
ConclusionsA new methodology to measure BCVA with a smartphone placed near the subject with an app that rescales the stimulus according to the face-device distance has been evaluated. A good agreement between the BCVA measured with the app with respect to clinical measures was found. This has the potential to facilitate home monitoring for adult patients; however, further research is needed to validate the use in children. The results have major implications in advancing telemedicine and providing ophthalmologists with a reliable and accessible method to measure VA and communicate results remotely between providers and patients.
We gratefully thank Philip Kruger for editing the original manuscript as well as the two anonymous reviewers for their constructive comments.