To determine the effectiveness of the MGDRx EyeBag in managing meibomian gland dysfunction.
MethodsThis was a prospective, randomized, controlled, observer-masked, bilateral eye study that enrolled 29 participants. Participants were randomized into either the EyeBag group or the control group. The EyeBag group used the EyeBag 10minutes 2x/day, and the control group remained on their own dry eye treatment regimen (if applicable). All participants were observed at baseline, 2 weeks (2wk) and 4 weeks (4wk). At 4wk, participants in the EyeBag group were asked to stop using the EyeBag. All participants were seen again at 8 weeks (8wk). Primary outcomes were the Ocular Surface Disease Index (OSDI), Current Symptoms Questionnaire (CSQ), meibomian gland score (MG score), and non-invasive tear breakup time (NIBUT).
ResultsTwenty-five participants completed the study (mean age 38±15 years, 7 male). There was a significant change in OSDI over time for the EyeBag group (mean[lower 95% CI, upper 95% CI], baseline: 39.1[31.1,47.0], 2wk: 26.8[19.7,33.9], 4wk: 26.6[16.5,36.7], 8wk: 27.7[18.4,37.0]; p=0.01), but not in the control group (p=0.22), but no significant difference between groups at all time points (all p>0.27). Symptoms immediately improved after conducting the EyeBag based on at-home CSQ scores (Δ=-5.0 points, p<0.01), but not in the control group. For both groups, there was no significant change (p-value EyeBag,p-value control) in MG score (0.21,0.17) and NIBUT (0.49,0.06) over time.
ConclusionsThe EyeBag may relieve symptoms of dry eye, but the effect on meibomian gland function and tear stability when used for only 4 weeks was undetectable.
Determinar la efectividad de MGDRx EyeBag en el tratamiento de la disfunción de las glándulas de Meibomio.
MétodosEstudio prospectivo, aleatorizado, controlado, simple ciego (investigador), bilateral, que incluyó a 29 participantes aleatorizados entre el grupo EyeBag o el grupo control. El grupo EyeBag utilizó dicho dispositivo 2 veces/día durante 10 minutos, y el grupo control siguió su propio tratamiento para ojo seco (de haberlo). Se observó a todos los participantes al inicio, a las 2 semanas (2sem) y 4 semanas (4sem). A las 4sem, se solicitó a los participantes del grupo EyeBag que interrumpieran el uso del dispositivo. Se volvió a examinar a todos los participantes a las 8 semanas (8sem). Los resultados primarios fueron el Índice de Enfermedad de la Superficie Ocular (IESO), el Cuestionario de Síntomas Actuales (CSQ), la clasificación de la glándula de Meibomio (puntuación MG), y NIBUT (non-invasive tear breakup time).
ResultadosVeinticinco participantes completaron el estudio (edad media 38±15 años, 7 varones). Se produjo un cambio significativo en IESO a lo largo del tiempo en el grupo EyeBag (media[IC inferior del 95%, IC superior del 95%], valor basal: 39,1[31, 1,47], 2sem: 26,8[19,7 33,9], 4sem: 26,6[16,5, 36,7], 8sem: 27,7[18,4, 37]; p=0,01), pero no en el grupo control (p=0,22), y no existió diferencia significativa entre grupos en todos los puntos temporales (total p>0,27). Los síntomas mejoraron de inmediato tras utilizar EyeBag, sobre la base de puntuaciones CSQ domiciliarias (Δ=-5 puntos, p<0,01), pero no en el grupo control. Para ambos grupos, no se produjo cambio significativo (valor p EyeBag, valor p control) en la puntuación MG (0,21, 0,17) y NIBUT (0,49, 0,06) con el tiempo.
ConclusionesEyeBag puede aliviar los síntomas del ojo seco, pero el efecto sobre la función de las glándulas de Meibomio y la estabilidad de la lágrima al utilizarse durante 4 semanas fue indetectable.
Meibomian glands (MGs) are modified sebaceous glands imbedded within the tarsal plate of the eyelids.1,2 The glands consist of multiple acini which manufacture and secrete meibum (oils) into a central duct. The duct terminates in an orifice at the eyelid margin posterior to Marx's line. The meibum released at the orifice serves to mix and stabilize the tear film.2,3
When the gland orifices become obstructed, the delivery of meibum into the tear film is reduced.1 The primary mechanism of gland orifice obstruction appears to be driven by hyperkeratinization and thickening of the MG ductule epithelium.4,5 This results in a loss of tear film stability3 and brings about symptoms of dry eye (burning, stinging, foreign body sensation).6 Symptoms of dry eye can range from mild to severe7 and can potentially have a large impact on economic burden8 and quality of life of the patient.9 This condition is termed meibomian gland dysfunction (MGD).7 As per the 2011 International Workshop on Meibomian Gland Dysfunction, the recommended definition of MGD is: “… a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. This may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.”10
Meibomian gland dysfunction is thought to be the leading cause of dry eye.7,11 The prevalence of MGD varies widely and have been reported to range from 3.5%12 to 69.3%,13 depending on ethnicity and the working definition of MGD used for study inclusion.14 The condition appears to be associated with, but not limited to, aging,15,16 taking certain medications (e.g. retinoids),17–20 androgen deficiency,21–23 and possibly contact lens wear.14,24,25
One of the mainstay treatments for MGD involves warm compresses.26,27 The application of heat and pressure to the eyelid margin melts the meibum plugging the gland orifices and facilitates delivery of meibum from the gland into the tear film.27 One standard treatment recommendation is to wet a face towel with hot water and apply the towel to the eyelid margins.28 This procedure has limitations, such as the inconvenience of constantly holding the towel against the face, rapid heat loss from the towel, and the constant need to replace or reheat the towel.29,30 Recently, a number of commercial products have been developed with the similar principle of delivering either moist or dry heat to the eyelids, and all of them attempt to retain heat more effectively than a face towel.29,31–35 The effectiveness of therapy is related to heating duration36 and the performance of different products may vary.37 The temperature of the device and the device-eyelid interface can be directly measured using thermometer probes to ensure that the device is working at operating temperatures.35,36,38,39
The MGDRx EyeBag (The EyeBag Company Ltd, Halifax, UK) is another product40 designed for the treatment of MGD. It is essentially a cotton/silk pouch filled with flax and linseed.41 The product retains heat well and can be easily fitted over the closed eyes without difficulty.29 To date, there are two other published studies examining the clinical treatment effect of the EyeBag on MGD.32,38 One study found an improvement in symptoms and MG function in the eye receiving treatment,32 and the other found improved tear stability and lipid layer grade.38 Both studies employed a contralateral eye design where the treatment and control were applied to eyes of the same individual and then simultaneously compared. The purpose of this current study is similar, in that it examines the effectiveness of the EyeBag in improving MG function and symptoms. The difference is in the study design (bilateral eye), which we feel allows for a more accurate representation of symptom change, and that this study also examines symptoms and signs after cessation of treatment.
MethodsParticipantsThis study was conducted in conformance with the ethical principles of the Declaration of Helsinki, the ICH guidelines for Good Clinical Practice, and the UW Guidelines for Research with Human Participants. Informed consent was obtained from all participants prior to enrollment in the study. Ethics clearance was obtained through a UW Research Ethics Committee prior to commencement of the study.
This was a prospective, randomized, controlled, observer-masked, bilateral eye study that enrolled 29 symptomatic dry eye participants. This study was conducted during the span of 5 months, between December 2014 - April 2015 at the Centre for Ocular Research and Education, at the University of Waterloo, School of Optometry & Vision Science (43.5°N, 80.5°W). All participants were sourced from the Waterloo community.
The sample size was determined using G*Power 3.1 (Universität Kiel, Kiel, Germany).42,43 The power analysis selected was an a-priori, two-tailed, matched pairs, t-test. To detect a 3.0 mean difference in meibomian gland score (MG score, see clinical methods for more details), with a common SD of 3.0 (1.0 effect size), at 80% power and 0.05 level of significance, a minimum sample size of 10 in each group was required. By factoring a 20% dropout rate in each group, the total number required to complete is 24. The 3.0 mean difference was selected since it was a modest value compared to the reported 7.0 mean difference previously reported with another device that has been developed for the management of MGD, the Johnson & Johnson LipiFlow.44
The key inclusion and exclusion criteria are outlined in Table 1.
Criteria for study entry.
| Inclusion | Exclusion |
|---|---|
| At least 17 years of age | Has a systemic condition or on medication that may affect an outcome variable (e.g. previous refractive and/or cataract surgery, glaucoma medications, Sjögren's syndrome, rheumatic disease) |
| Ocular Surface Disease Index ≥ 23 | Hypersensitivity to EyeBag components |
| Meibomian Gland Score ≤ 9 | |
| Not a contact lens wearer |
This study consisted of 4 visits. At the initial visit, participant eligibility was determined and baseline data was collected. Participants were randomized into either the EyeBag (treatment) or control group. The randomization of participants was conducted using Microsoft Excel 2010 (Redmond, Washington, USA). The order of preassigned participant IDs from the sample were randomized in a column using the rand() function. The participant IDs from the top half of the column were selected to be in the treatment group, and participants from the bottom half were selected to be in the control group. Participants in the control group were asked to remain on their current dry eye regimen and to keep medication dosages steady for the duration of the study period. Participants in the EyeBag group were provided EyeBags and were instructed to use them for 10minutes twice daily as per the enclosed brochure. All participants returned at 2 weeks and 4 weeks for follow-up assessments. After 4 weeks, participants in the EyeBag group ceased EyeBag use and all participants returned for a final assessment at the 8 week mark.
Clinical methodsAt the beginning of each follow-up study visit, a research assistant asked participants how often they had used the EyeBag at home. Participant compliance information regarding frequency and duration of use was documented. Changes to health or medications were also documented. All clinical tests were conducted by one investigator and in the same order each time, as they appear below.
Dry eye symptoms were measured using the Ocular Surface Disease Index (OSDI)45 and the Symptom Assessment iN Dry Eye (SANDE)46 questionnaires. The OSDI is a questionnaire that assessed severity of dry eye by querying symptoms, difficulties with certain tasks, and comfort in different environments.45 The SANDE consists of visual analogue scales that queried severity and frequency of dry eye symptoms.46
Tear film lipid layer thickness was conducted using the LipiView (Johnson & Johnson Vision, Santa Ana, California, USA) in primary gaze.47
High and low contrast visual acuity was assessed using an electronic computer LogMAR chart at an optical length of 6 meters. Participants were asked to read rows of letters that decreased in size until no more letters could be read.
Non-invasive tear breakup time was assessed by using the Humphrey Atlas Topographer 991 (Zeiss, California, USA). The topographer projected illuminated placido discs onto the cornea48 and the time for distortion to appear on the placido discs during the interblink interval was recorded 3 times in sequence and then averaged.
Corneal staining and conjunctival staining was assessed by wetting a fluorescein strip with a few drops of sterile saline, shaking off the excess, and instilling it in both eyes. Corneal and conjunctival staining each was assessed using the CORE scale (corneal: 0 to 100 for type, extent, and depth; conjunctival: 0 to 100),49 both with cobalt blue illumination through a Wratten no. 12 barrier filter.
Meibomian gland assessment was conducted using the Meibomian Gland Evaluator (Johnson & Johnson Vision, Santa Ana, California, USA).50 The leading edge was applied against the central inferior eyelid, exerting a pressure of 1.25g/mm2. The appearance of meibum expressed from the 5 central glands were assessed.44 The grading scale used was as follows; grade 0: no secretion, grade 1: inspissated, grade 2: turbid and particulate liquid, grade 3: clear oily liquid.51 The MG score is a sum of the grades from each of the 5 central glands.44 The Meibomian Glands Yielding Liquid Secretions (MGYLS) score is a count of the number of glands with MG score 2 or higher.
Meibography was assessed using the meibography feature on the Keratograph 5M (OCULUS, Wetzlar, Germany).52 Both the inferior and superior eyelids of both eyes were everted and imaged. Gland dropout was graded using the Arita scale53 and the final score for each eye was obtained by summing the grade from the upper and the lower eyelids.
During the study visit, participants in the EyeBag group received an EyeBag treatment. The EyeBag was heated in a 900W microwave (RCA, New York, USA) on high power for 30seconds and then placed over the participants’ closed eyes for 10minutes, according to manufacturer recommendation.40 The temperature profile of an EyeBag heated for 30seconds in a 900W microwave is detailed in Appendix 1. Digital massage of the eyelids was then conducted according to manufacturer instructions (i.e. a gentle sweeping motion across the eyelids in a nasal to temporal motion for 10 cycles). The Current Symptoms Questionnaire (CSQ) was conducted immediately before and after the EyeBag treatment. The CSQ is a subset of the Dry Eye Questionnaire and had been modified to assess immediate symptoms.54,55 Participants randomized into the control group did not receive the EyeBag treatment and was asked to complete the CSQ twice, 10minutes apart, with no intervention in between. Both high and low contrast visual acuity was recorded again immediately after EyeBag treatment.
At the end of the baseline visit, participants in the EyeBag group were provided the EyeBag to use at home. As per the commercial brochure that was included with the EyeBag, participants were instructed to heat the EyeBag in a microwave oven for 30s before placing it over their eyes for 10minutes. Participants were also instructed to conduct digital massage immediately after the 10minutes had elapsed, as per the brochure. They were not restricted to when they were required to use the EyeBag at home, however they must use it twice a day. Participants in the control group did not receive an EyeBag and were asked to remain on their own dry eye regimen (if applicable) for the duration of the study.
The CSQ was also administered at home via smartphone using the MetricWire app (Kitchener, Ontario, Canada).56 This app enabled participants to receive notifications and fill out the CSQ on their smartphone. Similar to above, the participants who were randomized into the EyeBag group were instructed to fill in the at-home CSQ via smartphone prior to using the EyeBag and then immediately after using the EyeBag. The participants in the control group were asked to fill out the at-home CSQ via smartphone twice, 10minutes apart, with no intervention in between. Participants were only required to do this once during weeks 1 and 3, so that there would be 2 pairs of pre/post at-home CSQ values per participant.
Statistical AnalysisStatistical analyses were conducted using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). Normal data distribution testing was conducted using the Shapiro-Wilk normality test at the level of α=0.05. Parametric data is presented as mean with 95% confidence intervals and nonparametric data is presented as medians with 95% confidence intervals.
To determine statistically significant change over time, repeated measures ANOVA with Bonferroni correction or Friedman test with Dunn's multiple comparisons test were used. Paired t-test or Wilcoxon test was used to determine differences between pre and post EyeBag CSQ, and HCVA/LCVA values. Mann-Whitney test was used to determine the difference in CSQ score reduction between the EyeBag and control group.
Level of statistical significance is at the level of α=0.05. Data from only the left eye (where applicable) was analyzed.
ResultsTwenty five participants completed the study (mean age 38±15 years, 7 male). Twelve were randomized into the EyeBag group (mean age 38±15 years, 3 male), 13 were randomized into the control group (mean age 37±15 years, 4 male). Two participants in the treatment group were using artificial tears regularly. Four participants were found to be ineligible for the study at the screening visit. Their data was not used in the analysis.
Adherence to TherapyParticipants were instructed to use the EyeBag twice daily for 10minutes each. On average, participants in the EyeBag group reported having used the EyeBag (mean±SD) 1.9±0.3 times a day, for 9.6±1.4minutes each, from baseline to week 2. From weeks 2 to 4, participants used the EyeBag 1.7±0.5 times per day for 9.6±1.4minutes each. Overall, participants in the EyeBag group reported having used the EyeBag 1.8 times per day for approximately 9.6minutes for the duration of the treatment period.
Clinical ResultsThere was a significant change in OSDI over time for the treatment group (Figure 1, left). For the EyeBag group, the mean score at baseline was 39.1[31.1, 47.0], which decreased significantly (-12.3[1.9, 22.6] points, p=0.02) to 26.8[19.7, 33.9] at week 2 and remained lowered for the duration of the study (26.6[16.5, 36.7] at week 4 (p=0.01), 27.7[18.4, 37.0] at week 8 (p=0.03)). For the control group, the OSDI score was 41.3[32.0, 50.6] at baseline and did not change significantly over time (p=0.22). Symptom reduction was also reflected in the SANDE score (Figure 1, right). The EyeBag group showed a reduction over time from baseline, with the maximum reduction observed at week 4 (-13.0[0.52, 25.4] points). The control group also showed change over time, however in the opposite direction. The baseline control SANDE score was 52.4[42.9, 61.8] and increased to 63.0[44.5, 81.6] over the duration of the study. None of these changes were statistically significant (p=0.25 in the EyeBag, p=0.15 in the control). When comparing between the two groups, there were no significant differences in OSDI and SANDE scores between the EyeBag and the control group at all time points. The difference in OSDI scores (EyeBag-control) were -2.3 [-19.5, 15.0) at baseline (p=0.99), -12.1 [-29.4, 5.1] at week 2 (p=0.27), -9.2 [-26.4, 8.1] at week 4 (p=0.54), and -7.9 [-25.2, 9.3] at week 8 (p=0.68). The difference in SANDE scores (EyeBag-control) were 6.4 [-18.3, 30.9] at baseline (p=0.94), -7.8 [-32.4, 16.8] at week 2 (p=0.89), -17.1 [-41.7, 7.5] at week 4 (p=0.28), -13.6 [-38.2, 11] at week 8 (p=0.51).
Summary (mean[lower 95% CI, upper 95% CI]) of OSDI (left) and SANDE (right) changes over time. The OSDI scores in the EyeBag group were significantly different than baseline at weeks 2 (-12.3[1.9, 22.6], p=0.02) and 4 (-12.5[2.1, 22.0], p=0.01), and 8 (-11.4[1.0, 21.7], p=0.03). There was no significant change over time for the control group (p=0.22). There was no significant difference over time in either the EyeBag (p=0.25) or control group (p=0.15) for the SANDE scores.
The median MG score for the EyeBag group increased from 4.0[2.0, 7.0] to 6.0[3.0, 8.0] points from baseline to week 2, 5.0[3.0, 7.0] at week 4, and returned to 4.0[2.0, 6.0] at week 8. None of these changes were statistically significant (p=0.21). For the control group, the median MG score stayed at 3.0[0.0, 6.0] from baseline to week 2 (3.0[2.0, 5.0]). This decreased to 1.0[0.0, 5.0] at week 4 and 1.0[0.0, 6.0] at week 8. The changes were also not statistically significant (p=0.17) (Figure 2, left). The median EyeBag MGYLS scores increased from 1.5[0.0, 3.0] at baseline to 2.0[1.0, 3.0] at weeks 2 and 4, and decreased to 0.0[0.0, 2.0] at week 8. In the control group, median MGYLS scores stayed at 0.0 for the entire study duration (Figure 2, right). None of the changes in either group were statistically significant (p=0.14 for EyeBag, p=0.40 for control).
The medians[lower 95% CI, upper 95% CI] of MG score (left) and MGYLS (right) over time. An overall improvement in median MG score and MGYLS was observed by 4 weeks relative to baseline (+1.0 units, +0.5 glands, respectively) and appeared to return to baseline levels at week 8. None of these changes were statistically significant (p=0.21 for MG score, p=0.14 for MGYLS) in the EyeBag group, and the control group (p=0.17 for MG score, p=0.40 for MGYLS).
For NIBUT, a gradual increase was observed in the EyeBag group. At baseline, NIBUT was observed to be 2.9s[2.1s, 3.7s]. This value increased to 3.1s[2.6s, 3.6s] at 2 weeks, and 3.4s[2.9s, 3.9s] at 4 weeks, before decreasing to 3.0s[2.4s, 3.5s] at week 8. In the control group, NIBUT decreased from 3.1s[2.4s, 3.9s] at baseline to 2.7s[2.2s, 3.2s] at week 2, 2.3s[1.9s, 2.7s] at week 4 and 3.1s[2.5s, 3.7s] at week 8. Neither groups showed statistically significant changes (p=0.49 for EyeBag, p=0.06 for control). Figure 3 summarizes the change in NIBUT.
Non-invasive tear breakup time (mean[lower 95% CI, upper 95% CI]) in the EyeBag group increased gradually from baseline to 4 weeks (+0.5s[-0.4s, 1.2s], p=0.51) but returned to baseline levels at week 8 (0.0s[-0.8s, 0.8s], p=0.99]. None of the changes in the EyeBag (p=0.49) or control group (p=0.06) were statistically significant.
There was a statistically significant difference detected in conjunctival staining over time, however it was not likely to be clinically significant, as a reduction of 4.0 points on a scale of 100 was relatively minor. Furthermore, there was no statistically significant change in corneal staining, lipid layer thickness, and meibography for both the EyeBag and control group (all p>0.05). Tables 2 and 3 summarize the clinical results for the EyeBag group and control group respectively.
Summary of clinical changes for the EyeBag group (n=12).
| Baseline | 2 weeks | 4 weeks | 8 weeks | p value | |
|---|---|---|---|---|---|
| Parametric (mean[lower 95% CI, upper 95% CI]) | |||||
| OSDI | 39.1[31.1, 47.0] | 26.8[19.7, 33.9]* | 26.6[16.5, 36.7]* | 27.7[18.4, 37.0]* | 0.02 |
| SANDEb | 58.7[49.5, 67.9] | 50.1[33.9, 66.4] | 45.8[28.5, 63.0] | 49.4[29.2, 69.5] | 0.25 |
| LLT | 69[58,79] | 68[59,77] | 64[57,72] | 64[56,73] | 0.13 |
| NIBUT (seconds) | 2.9[2.1, 3.7] | 3.1[2.6, 3.6] | 3.4[2.9, 3.9] | 3.0[2.4, 3.5] | 0.49 |
| Meibography | 2.2[1.3, 3.1] | 2.3[1.4, 3.3] | 2.2[1.4, 3.1] | 2.2[1.3, 3.1] | 0.53 |
| Non parametric (median[lower 95% CI, upper 95% CI]) | |||||
| MG Scorea | 4.0[2.0, 7.0] | 6.0[3.0, 8.0] | 5.0[3.0, 7.0] | 4.0[2.0, 6.0] | 0.21 |
| MGYLSa | 1.5[0.0, 3.0] | 2.0[1.0, 3.0] | 2.0[1.0, 3.0] | 0.0[0.0, 2.0] | 0.14 |
| Corneal staininga | 30.0[10.0, 115.0] | 25.0[0.0, 100.0] | 62.5[0.0, 150.0] | 62.5 [0.0, 125.0] | 0.95 |
| Conjunctival staininga | 4.0[2.5, 11.2] | 0.0[0.0, 1.2]* | 6.3[3.8, 15.0] | 0.0[0.0, 2.5]* | <0.01 |
Summary of clinical changes for the control group (n=13).
| Baseline | 2 weeks | 4 weeks | 8 weeks | p value | |
|---|---|---|---|---|---|
| Parametric (mean[lower 95% CI, upper 95% CI]) | |||||
| OSDI | 41.3[32.0, 50.6] | 39.0[28.2, 49.7] | 35.8[23.2, 48.3] | 35.6[21.6, 49.7] | 0.22 |
| SANDEb | 52.4[42.9, 61.8] | 57.9[46.1, 69.7] | 62.9[49.0, 76.8] | 63.0[44.5, 81.6] | 0.15 |
| NIBUT | 3.1[2.4, 3.9] | 2.7[2.2, 3.2] | 2.3[1.9, 2.7] | 3.1[2.5, 3.7] | 0.06 |
| Meibography | 2.2[1.3, 3.2] | 2.2[1.2, 3.2] | 2.2[1.3, 3.0] | 1.8[0.9, 2.6] | 0.16 |
| Non Parametric (median[lower 95% CI, upper 95% CI]) | |||||
| MG Scorea | 3.0[0.0, 6.0] | 3.0[2.0, 5.0] | 1.0[0.0, 5.0] | 1.0[0.0, 6.0] | 0.17 |
| MGYLSa | 0.0[0.0, 3.0] | 0.0[0.0, 2.0] | 0.0[0.0, 2.0] | 0.0[0.0, 1.0] | 0.40 |
| LLTa | 74[57,87] | 67[55,76] | 56[53,67] | 60[52,77] | 0.07 |
| Corneal staininga | 10.0[0.0, 25.0] | 25.0[0.0, 50.0] | 0.0[0.0, 25.0] | 0.0[0.0, 50.0] | 0.37 |
| Conjunctival staininga | 1.2[0.0, 5.5] | 0.0[0.0, 2.5] | 2.2[0.5, 13.8] | 0.0[0.0, 1.2] | <0.01 |
The pre and post EyeBag CSQ scores conducted at CORE were pooled from all study visits for analysis (n=48 for the EyeBag group, n=52 for the control group). The EyeBag and control group both showed a statistically significant decrease in CSQ scores (mean difference of -5.5[-6.9, -3.9] points for the EyeBag, -2.5[-3.4, -1.6] points for the control, both p<0.05 by Paired t-test) over the course of 10minutes. The decrease in symptoms in the control group was likely describing recovery from discomfort induced by clinical testing. After applying an offset (+2.5 points) to the post-EyeBag CSQ values to correct for clinically induced discomfort, the decrease in symptoms detected in the EyeBag group was still statistically significant. There was no change for both HCVA and LCVA in both groups (Table 4).
Paired t-test comparison of pooled visual acuities and at-CORE CSQ scores immediately pre and post EyeBag (after offset) application. All values are expressed as means [lower 95% CI, upper 95% CI limit].
| EyeBag group (n=48) | Control group (n=52) | |||||
|---|---|---|---|---|---|---|
| HCVA | LCVA | CSQ | HCVA | LCVA | CSQ | |
| Pre Eyebag | -0.04[-0.07, -0.02] | 0.21[0.17, 0.24] | 15.0[12.7, 17.3] | 0.00[-0.03, 0.04] | 0.31[0.26, 0.36] | 17.3[15.0, 19.7] |
| Post Eyebag | -0.05[-0.08, -0.02] | 0.19[0.16, 0.23] | 12.0[10.2, 13.8] | 0.01[-0.03, 0.04] | 0.29[0.24, 0.33] | 17.3[15.3, 19.5] |
| P value | 0.11 | 0.11 | <0.01 | 0.82 | 0.09 | 1.00 |
Participants in the EyeBag group were instructed to complete the at-home CSQ immediately prior to and after the EyeBag treatment (10minutes). With n=12 in the EyeBag group we had expected a total of 24 pairs (pre/post) of responses (12 pairs for week 1, and another 12 for week 3). Due to some participants who had not completed the at-home CSQ properly, only 19 pairs of data were useable (9 pairs from week 1, 10 pairs from week 3). The median time between the submission of the first at-home CSQ, and the initiation of the second at-home CSQ was 22.6minutes (Q1: 13.5 mins, Q3: 61.6 mins), ranging from 0.6 mins to 289.1. The pre-EyeBag median [lower 95% CI, upper 95% CI] was 16.0[6.0, 26.0] and the post-EyeBag median [lower 95% CI, upper 95% CI] was 7.0[4.0, 13.0]. The resulting median of differences was -5.0[-9.0, -1.0] and was statistically significant (by Wilcoxon test, p<0.01). This study did not find a statistically significant correlation between treatment time and CSQ score reduction (Spearman's r=0.14, p=0.55).
In the control group, of the 26 pairs of responses, only 13 pairs of data were usable. The median time between at-home CSQ submissions was 13.5minutes (Q1: 9.1 mins, Q3: 22.7 mins) ranging from 1.3 mins to 37.4 mins. The median pre CSQ score was 8[5.0, 20.0] and the median post CSQ score was 9.5[4.0, 25.0]. The resulting median of differences was +1.0[-1.0, 3.0], which was not statistically significant (by Wilcoxon test, p=0.24). The median reduction in at-home CSQ scores in the EyeBag group was significantly greater than the control group, with differences between medians of 5.0[3.0, 10.0] (p<0.01 by Mann Whitney test).
DiscussionThe results from this study suggest that the changes in measurable signs from using the EyeBag were modest. While not statistically significant, almost all the variables in the EyeBag group changed in the direction that favoured treatment. In contrast, the numbers in the control group stayed the same or moved in the opposite direction.
A one point increase in MG score from baseline to the second week suggests that, on average, one of five glands showed an improvement in meibum grade. This change was maintained up to the fourth week but returned to baseline after discontinuing the EyeBag. This trend was also observed with NIBUT, where an improvement was observed during the period in which the EyeBag was used, and returning to baseline where it was discontinued. This suggests that the EyeBag needs to be used continuously to maintain the improvement in MG function and NIBUT. The potential changes in these clinical measures after extended use remains unknown.
The statistically non-significant findings with MG score and NIBUT may be due to an over-estimation of the impact of the EyeBag when calculating sample size. A post-hoc analysis showed that the effect size of the MG score in this study was actually around 0.4, which was much less than what the study was powered to detect (1.0). One reason that may explain a depressed effect size could be related to the dry winter environment during which this study was conducted. Desiccating environments challenge the ocular surface,57 and different climates have been found to have an impact on tear film testing.58 Therefore, to detect a statistically significant difference, future studies using this same study design and analysis would require at least n=52 per group. The two other EyeBag studies32,38 that found a statistically significant improvement with tear stability38 and MG score32 both had used a contralateral-eye design which enabled more statistical power to detect smaller differences.
Despite modest changes in MG score and NIBUT, both short (CSQ) and long-term (OSDI) symptoms improved significantly over time after using the EyeBag. Improvement over time was noticed as soon as week 2, and the OSDI remained decreased for the duration of the study, even after the EyeBag was discontinued. However, likely due to the small sample size, it was not possible to uncover symptom differences between the EyeBag group and control group. Furthermore, it is not clear why symptoms remained decreased when both MG score and NIBUT appeared to return to baseline after discontinuing use. There may have been a placebo effect,59 in which participants believed they felt better after having undergone the EyeBag therapy. Regardless, the OSDI score reduction was not as high as reported by the previous EyeBag study32 and we think this difference may be due to bias inherent with a contralateral design and the application of the questionnaire in a unilateral manner.
Short term symptom reduction measured with the CSQ was also considered to be minor. A 3.0 point (at-CCLR) and 5.0 point (at-home) CSQ score reduction is considered to be small on a scale out of 50. It was also not possible to predict symptom improvement as there was no observable correlation between treatment time and CSQ symptom reduction.
Meibomian gland atrophy did not change significantly throughout the study. This was not surprising, since it takes many years for MG atrophy to occur.53 Due to this fact, we also could not conclude whether or not the EyeBag prevents or reverses atrophy. Furthermore, we did not find any clinically meaningful changes with corneal staining, conjunctival staining and lipid layer thickness.
Due to the importance of adherence in medical therapy60 this study had attempted to quantify EyeBag use with two methods (self-reporting to research assistant, and via MetricWire app). Although participants self-reported using the EyeBag 1.8 times a day for 9.6minutes, the timestamps from the CSQs administered via the MetricWire app suggested differently. We could only conclude that participants were either not completing the CSQs in the instructed manner or they were using the EyeBag differently from what they reported. At the end of the study, some participants mentioned they had fallen asleep while using the EyeBag, while some others mentioned that 10minutes was too time consuming. Without any more information, it is not possible to make any further conclusions with EyeBag compliance.
All participants have different microwave ovens so it was difficult to determine if all EyeBags were heated to the same extent. There also appears to be variation in EyeBag temperature retention between EyeBags (Appendix 1) after being heated with the same amount of power. This may have contributed to variability in treatment efficacy.
The EyeBag is aimed towards managing obstructive MGD by melting and softening meibum obstructing the MGs. However, various forms of MGD exist, and not all are necessarily driven by obstruction.61 Also, it is currently unclear whether or not atrophied glands may benefit from the EyeBag treatment. This may be an area for future research. Additionally, in severe cases of MGD, eyelid warming devices may not be enough for treatment. Blepharitis is often associated with MGD62 and it may be beneficial to study the combined effect of an eyelid warming device with antibiotics or anti-inflammatory therapies. Additionally, since there are many emerging eyelid warming devices, it may also be beneficial to compare their efficacies in the management of MGD.
ConclusionThere was a considerable improvement in long and short term symptoms after using the MGDRx EyeBag, but a placebo effect cannot be ruled out. Changes in MG function and tear breakup time were not detectable in this study. The continued use of the EyeBag is required to sustain improvement, but given that symptoms did improve, then compliance may be helped by the fact that participants do seem to appreciate a reduction in symptoms.
FundingThis study was funded by the Centre for Ocular Research & Education (CORE) and the Canadian Optometric Education Trust Fund
Conflicts of InterestMembers of the Centre for Ocular Research & Education have received funding/honoraria from the following companies over the past 3 years; Alcon, Allergan, Contamac, CooperVision, Essilor, GL Chemtec, Inflamax Research, J&J Vision, Menicon, Nature's Way, Novartis, Safilens, Santen, Shire, SightGlass, TearLab, TearScience and Visioneering.
The authors want to acknowledge the following people for their contribution to the paper. Brian Stewart and Charles Desouza for their technical support with the MetricWire system. Damaris Grau for her contribution of the EyeBag technical data. The EyeBag Company Ltd for their generous supply of EyeBags.
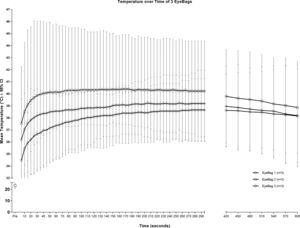
An EyeBag was heated in 900W microwave (RCA, USA) for 30seconds according to manufacturer instructions.41 After removal from the microwave, the EyeBag was lightly shaken to evenly distribute the heated seeds. The silver side (silk) was set down flat against a wooden surface and centered on top of a HH-20A series digital thermometer probe (Omega Engineering, Stamford, Connecticut, USA). The temperature of the EyeBag was recorded every 5seconds for the first 5minutes and every 30seconds for the subsequent 5minutes, for a total duration of 10minutes. This was repeated two more times and the mean temperature for each time point was recorded. This procedure was repeated with another 2 separate EyeBags.
After removal from the microwave, the temperatures of the EyeBags continued to slowly climb. The peak temperature of each EyeBag occurred at approximately 3minutes before slowly decaying over the course of 10minutes. The maximum for EyeBag 1, 2 and 3 was 38.7°C [37.5°C, 39.8°C] @ 4:35 mins, 40.4°C [38.3°C, 42.5°C] @ 2:45 mins, and 39.2°C [38.2°C, 40.2°C] @ 3:25 mins, respectively. Mean temperatures for EyeBag 1, 2, and 3 at the end of the 10minute period was 38.2°C [36.2°C, 40.1°C], 38.9°C [37.1°C, 40.6°C], and 38.2°C [36.4°C, 40.0°C] respectively.
A two-way ANOVA with post-hoc Tukey's multiple comparison test was used to determine the statistical significance of the difference between the EyeBags at different time points.
Prior to microwaving (t=Pre), there was no significant difference between all 3 EyeBags. However, EyeBag 1 and EyeBag 2 were significantly different between t=5s and t=80s inclusive, with maximum difference of 3.3°C occurring at the t=20s mark. EyeBags 2 and 3 were not significantly different from each other at all time points. EyeBags 1 and 3 were not significantly different from each other at all time points. Figure 4 is a temperature-time curve highlighting the temperature retention of each EyeBag.
Mean temperature-over-time curves of 3 different EyeBags. Temperatures continued to quickly rise for the first 60s before slowly plateauing and decaying slowly. At the end of the 600s duration, the temperature of all EyeBags were at least 38.1°C. EyeBag 3 was not significantly different than EyeBags 1 or 2 at all time-points. EyeBag 1 and 2 was significantly different only between t=5s and t=80s.










![Summary (mean[lower 95% CI, upper 95% CI]) of OSDI (left) and SANDE (right) changes over time. The OSDI scores in the EyeBag group were significantly different than baseline at weeks 2 (-12.3[1.9, 22.6], p=0.02) and 4 (-12.5[2.1, 22.0], p=0.01), and 8 (-11.4[1.0, 21.7], p=0.03). There was no significant change over time for the control group (p=0.22). There was no significant difference over time in either the EyeBag (p=0.25) or control group (p=0.15) for the SANDE scores. Summary (mean[lower 95% CI, upper 95% CI]) of OSDI (left) and SANDE (right) changes over time. The OSDI scores in the EyeBag group were significantly different than baseline at weeks 2 (-12.3[1.9, 22.6], p=0.02) and 4 (-12.5[2.1, 22.0], p=0.01), and 8 (-11.4[1.0, 21.7], p=0.03). There was no significant change over time for the control group (p=0.22). There was no significant difference over time in either the EyeBag (p=0.25) or control group (p=0.15) for the SANDE scores.](https://static.elsevier.es/multimedia/18884296/0000001200000002/v1_201904050614/S1888429618300815/v1_201904050614/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w90elkTtpMHXMkN9jatH7+0Y=)
![The medians[lower 95% CI, upper 95% CI] of MG score (left) and MGYLS (right) over time. An overall improvement in median MG score and MGYLS was observed by 4 weeks relative to baseline (+1.0 units, +0.5 glands, respectively) and appeared to return to baseline levels at week 8. None of these changes were statistically significant (p=0.21 for MG score, p=0.14 for MGYLS) in the EyeBag group, and the control group (p=0.17 for MG score, p=0.40 for MGYLS). The medians[lower 95% CI, upper 95% CI] of MG score (left) and MGYLS (right) over time. An overall improvement in median MG score and MGYLS was observed by 4 weeks relative to baseline (+1.0 units, +0.5 glands, respectively) and appeared to return to baseline levels at week 8. None of these changes were statistically significant (p=0.21 for MG score, p=0.14 for MGYLS) in the EyeBag group, and the control group (p=0.17 for MG score, p=0.40 for MGYLS).](https://static.elsevier.es/multimedia/18884296/0000001200000002/v1_201904050614/S1888429618300815/v1_201904050614/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w90elkTtpMHXMkN9jatH7+0Y=)
![Non-invasive tear breakup time (mean[lower 95% CI, upper 95% CI]) in the EyeBag group increased gradually from baseline to 4 weeks (+0.5s[-0.4s, 1.2s], p=0.51) but returned to baseline levels at week 8 (0.0s[-0.8s, 0.8s], p=0.99]. None of the changes in the EyeBag (p=0.49) or control group (p=0.06) were statistically significant. Non-invasive tear breakup time (mean[lower 95% CI, upper 95% CI]) in the EyeBag group increased gradually from baseline to 4 weeks (+0.5s[-0.4s, 1.2s], p=0.51) but returned to baseline levels at week 8 (0.0s[-0.8s, 0.8s], p=0.99]. None of the changes in the EyeBag (p=0.49) or control group (p=0.06) were statistically significant.](https://static.elsevier.es/multimedia/18884296/0000001200000002/v1_201904050614/S1888429618300815/v1_201904050614/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w90elkTtpMHXMkN9jatH7+0Y=)