Dry eye syndromes can involve both nociceptive and neuropathic symptoms. Nociceptive symptoms are the normal physiological responses to noxious stimuli. Neuropathic symptoms are caused by a lesion or disease of the somatosensory nervous system and can be the result of hypersensitisation of peripheral or central corneal and conjunctival somatosensory nerves. For example, inflammation could induce neuroplastic peripheral sensitisation of the ocular surface or lid wiper and exacerbate nociceptive symptoms. Neuropathic symptoms may explain the incommensurate relation between signs and symptoms in some dry eye syndromes although absence of signs of a dry eye syndrome may also be a consequence of inappropriate methods used when examining for them. Involvement of neuropathic mechanisms may also help explain dry eye symptoms which occur in association with reduced corneal sensitivity. This review includes a discussion of the potential for ocular symptoms involving neuropathic mechanisms to contribute to psychosocial problems such as depression, stress, anxiety and sleep disorders as well as for these types of psychosocial problems to contribute to neuropathic mechanisms and dry eye syndromes. Failure to consider the possibility that neuropathic mechanisms can contribute to dry eye syndromes may reduce accuracy of diagnosis and the suitability of treatment provided. Dry eye symptoms in the absence of commensurate evidence of tear dysfunction, and unsatisfactory response to tear dysfunction therapies should prompt consideration of neuropathic mechanisms being involved. Symptoms which persist after local anaesthetic instillation are more likely to be neuropathic in origin. Reducing inflammation may help limit any associated neuroplastic hypersensitivity.
Los síndromes de ojo seco pueden implicar síntomas tanto nociceptivos como neuropáticos. Los síntomas nociceptivos son las respuestas fisiológicas normales a los estímulos nocivos. Los síntomas neuropáticos son causados por una lesión o enfermedad del sistema somatosensorial, y pueden ser resultado de una hipersensibilización de los nervios sensoriales periférico o central de la córnea, o de la conjuntiva. Por ejemplo, la inflamación podría inducir una sensibilización periférica neuroplástica de la superficie ocular, o una epilopatía del párpado en limpiaparabrisas, o exacerbar los síntomas nociceptivos. Los síntomas neuropáticos pueden explicar la enorme relación entre los signos y síntomas en algunos síndromes del ojo seco, aunque la ausencia de signos en dichos síndromes puede ser también consecuencia de los métodos inapropiados utilizados al examinar dichos ojos. La implicación de mecanismos neuropáticos puede ayudar también a explicar los síntomas del ojo seco que se producen junto con la reducción de la sensibilidad corneal. Esta revisión incluye una discusión sobre el potencial de que los síntomas oculares que implican mecanismos neuropáticos contribuyan a los problemas psicosociales tales como depresión, estrés, ansiedad y trastornos del sueño, así como que dichos tipos de problemas psicosociales puedan contribuir a los mecanismos neuropáticos y los síndromes del ojo seco. La ausencia de consideración de la posibilidad de que los mecanismos neuropáticos puedan contribuir a los síndromes del ojo seco puede reducir la precisión del diagnóstico y la idoneidad del tratamiento suministrado. Los síntomas del ojo seco, en ausencia de evidencia conmensurable de disfunción lagrimal, y respuesta insatisfactoria a las terapias de disfunción lagrimal, deberían impulsar la consideración de una implicación de los mecanismos neuropáticos. Es probable que la persistencia de los síntomas tras la instilación de anestésicos locales tenga un origen neuropático. Reducir la inflamación puede ayudar a limitar cualquier hipersensibilidad neuroplástica asociada.
Based on the definition adopted by the 2007 International Dry Eye Workshop1 dry eye syndromes (DES) are multifactorial diseases of the tears, lids and ocular surface which can result in symptoms of discomfort and/or visual disturbance and/or tear film instability with the potential for damage to the ocular surface. They are usually accompanied by Meibomian gland dysfunction, increased osmolarity of the tear film and inflammation of the ocular surface1 and for example, may be exacerbated by infrequent and/or incomplete blinking.2 DES may be predominantly symptomatic but without obvious signs of ocular surface disease as well as presenting with evidence of ocular surface disease without significant symptoms.1,3 Dry eye symptoms represent nonspecific pain4 with the most consistent clinical feature of dry eye disease being chronic dry eye-like pain.5 Chronic pain syndromes are common in dry eye disease patients and are associated with increased severity of dry eye disease symptoms even though objective ocular surface signs are no worse6 or even non-existent. It may be assumed that ocular irritation is a painful stimulus7 so that many people with mild to moderate dry eye disease describe their symptoms as irritating rather than painful. However, many patients with a DES describe features of neuropathic pain,8 sometimes presenting without signs of epitheliopathy (pain without stain).9 Patients with more moderate symptoms arising from neuropathic mechanisms may present with symptoms of irritation without corresponding signs (irritation without desiccation for example).
However, a clinical finding of absence of signs of tear dysfunction may need to be qualified by the method of examination used. For example, a single instillation of fluorescein dye may not be sufficient to elicit evidence of ocular surface or lid wiper epitheliopathy associated with desiccation10 Sequential instillations of appropriate concentrations of more than one dye may, over time, elicit staining of the ocular surface or lid wiper which was not previously evident.11 The volume and concentration of stains instilled are additional variables and, for example, the type and manufacturer of dye impregnated strips could be important.12 Further scope for qualifying observations is obtained by the use of barrier filters which improve the ability to detect staining.13 A finding of ‘no corneal stain’ after a single instillation of one type of staining agent may be more correctly phrased as ‘no corneal stain detected’. Multiple instillations may compromise the epithelium but a second instillation could be sufficient to manifest staining which indicates a deficient epithelial barrier function not detected with an initial instillation. Emphasis on corneal changes and failure to examine for conjunctival14 and lid wiper epitheliopathy11 may also contribute to under-assessment of signs of dry eye. Failure to detect evidence of tear hyperosmolarity, deficient aqueous tear production or Meibomian gland dysfunction for example, could be other reasons why signs and other evidence of a DES are underestimated or missed.
Lack of correlation between signs and symptoms in DES may also be due to the symptoms not being a result of tear dysfunction or only partly being a result of tear dysfunction.15 For example, minimal signs of a DES or their absence may also be a consequence of symptoms arising from neuropathic mechanisms.9 A December 2015 PubMed search for “ocular neuropathy and dry eye” and for “neuropathic symptoms and dry eye” yielded 39 and 33 articles respectively. These and related publications were gleaned for information relevant to this review of dry eye symptoms.
Nociceptive symptomsThe occurrence of symptoms in a DES implies the activation of sensory nerves subserving nociception at the ocular surface.16 Candidate mechanisms for sensory nerve activation include tear film break-up during the interblink interval, tear and ocular surface hyperosmolarity, blink-related shear stress between the lids and globe in response to reduced tear volume and/or reduced expression of mucins at the ocular surface, the presence of inflammatory mediators at the surface of the eye, and hypersensitivity of the nociceptive sensory nerves.1 Depending on the relative activation by a stimulus of each subpopulation of corneal sensory fibres (mechanical, polymodal and cold), different sub-qualities of irritation and pain sensations are evoked.16
Although irritation and pain are the primary, and possibly the only sensations evoked by stimulation of the cornea, not all sensory neurons that innervate the cornea should be considered as nociceptors.17 For example, through the activation of transient receptor potential M8 channels,18 innocuous stimulation with mild cooling activates corneal afferents and induces tearing without causing irritation or pain.5 That cold receptor activation appears to evoke secretion of tears without accompanying pain18 is supported by the finding that they are the only corneal primary afferent neuron with spontaneous activity at room temperature.19 Sensations evoked by mechano- and polymodal nociceptors always include an irritation component.4 Symptom descriptors used by patients vary widely, possibly in part due to variable activation of different types and combinations of corneal nociceptors as well as in response to any contributions from neuropathic mechanisms.
Symptoms caused by tear dysfunction are more likely to be associated with signs such as ocular surface staining, tear and ocular surface hyperosmolarity, short tear film breakup times, the presence of inflammatory mediators at the ocular surface1 and lid wiper epitheliopathy.11 For example, in patients with symptomatic severe conjunctivochalasis, an increase in tear osmolarity was found to be associated with shorter tear break-up times and lissamine green stain.20
Neuropathic symptomsHowever there is another class of patients with dry eye symptoms who do not have commensurate signs. Patients diagnosed with dry eye may describe features of neuropathic pain, including spontaneous pain, dysesthesias (unpleasant abnormal sensations), hyperalgesia (exaggerated pain response to suprathreshold noxious stimuli), and allodynia (pain response to normally non-noxious stimuli21 such as wind and light). The terms allodynia and hyperalgesia which are often used interchangeably to refer to pain occurring in response to low intensity, non-tissue damaging, and normally non-painful stimulation, are ambiguous as to the afferent origin of that pain.22 Frequently, allodynia is presumed to be due to the activation of sensitised nociceptors by normally innocuous stimuli.22 Although this is sometimes the case, pain or irritation in allodynia is often due to the activation of normal non-nociceptive afferents operating upon a damaged or sensitised central nervous system.22 Current terminology is often ambiguous as to whether nociceptive or non-nociceptive afferent systems or both, are involved in pain perception.22
Terms used synonymously or in relation to neuropathic mechanisms include neuropathy, neuropathic pain, chronic pain, peripheral sensitisation, allodynia, hyperalgesia, hyperaethesia, neuralgia and neuroplastic changes. Notwithstanding distinctions between the meanings of these terms (some of which are subtle), the terms included in this review are as used in the publications in which they are cited. Neuropathic pain (that is pain caused by a lesion or disease of the somatosensory nervous system) results from damage and/or hypersensitisation of peripheral or central nervous system corneal and conjunctival somatosensory nerves (peripheral and central sensitisation).23 This definition includes disease processes such as inflammation and infers neuropathic pain involving an aberrant somatosensory processing which goes beyond the normal plasticity of the undamaged nociceptive system.24
The dynamic responsiveness of central sensitisation to increased peripheral pain activity triggered by nerve injuries and inflammation is a function of the remarkable neuroplasticity of the somatosensory nervous system.5 Neuroplasticity is also a fundamental mechanism of habituation25 or neuronal adaptation. For example, beneficial neuroplasticity can occur through peripheral and/or central nervous system desensitisation during adaptation or habituation to the initial discomfort of rigid contact lenses. Reduced corneal sensitivity in DES26 may be at least partly a function of neuroplastic hyposensitisation.
A Neurological Clinic study of patients with peripheral neuropathy associated with sicca complex found that although peripheral neuropathy was the presenting problem in 87%, sicca symptoms were reported by 93%.27 Sicca complex is the key feature of Sjogren's syndrome involving mononuclear infiltration and destruction of lacrimal and salivary glands (xeroophthalmia and xerostomia).27 However, the sicca symptoms were a presenting complaint in only 11% and were usually mild.27 These findings suggest that cases with more prominent sicca symptoms would follow a different referral path (ophthalmological or optometrical rather than neurological). However, compared to cases involving debilitating pain symptoms, these findings show that neuropathic sicca syndromes can involve a wide range of sicca symptom intensity.27
Some differences between neuropathic and nociceptive symptomsApart from nociceptive irritation or pain, which are the normal physiological responses to noxious stimuli, neuropathic pain may be involved in a DES and can become chronic as well as persist in the absence of the initiating insult.28 Nociceptive pain is often associated with tissue damage and a normal nervous system.29 However, neuropathic pain is associated with dysfunction of the physiological nervous system29 which is not necessarily associated with ocular tissue damage. Not infrequently, both of these pain types coexist.29 Nociceptive (physiological) pain is proportional to the stimulus and the associated nociception dominates consciousness but rapidly dissipates on removal of the stimulus.9 Consequently, physiological nociception normally recedes with repair of damaged tissue and resolution of inflammation.3 However intense and lengthy peripheral nociceptive input can cause central nervous system sensitisation so that irritation or pain continues as an autonomous activity in the form of neuropathic irritation or pain which is the pathology of neuralgia.3 Local anaesthetics do not always reduce corneal pain in which cases the pain may be explained by central nervous system sensitisation.30 Dry eye-associated ocular pain can be transient in some patients whereas others complain of chronic pain.21 Variation in exposure to environmental conditions may help explain transient symptoms for example. Pre-occupation with top-down cognitive tasks may reduce awareness of symptoms, especially those which are mild to moderate.31 Conversely, fatigue and reduced levels of demand from top-down activity may make it harder to maintain goal-relevant attention and the associated ability to inhibit symptoms which may not otherwise be as prominent.31
Hyperosmolarity and inflammatory contributions to symptomsThe unique location of the free corneal nerve endings between the superficial epithelial cells, very near to the ocular surface, makes them vulnerable to repeated damage from environmental exposures such as tear evaporation and pollution toxicity.21,32 Tear hyperosmolarity is regarded as the central mechanism causing ocular surface inflammation, damage, symptoms and the initiation of compensatory events in dry eye.1 Short tear breakup times and associated greater tear volume loss by evaporation are assumed to be the basis for hyperosmolarity.1 For example, automated tear film breakup time measured using an E300 corneal topographer, (Medmont International Pty. Ltd., Australia) was found to be a highly sensitive and highly specific clinical marker for tear osmolarity.33 Hyperosmolarity stimulates a cascade of inflammatory events in the epithelial surface cells, which arise from or activate inflammatory cells at the ocular surface.1
Activated corneal nerves release neuropeptides (neurogenic inflammation) which contribute to other forms of inflammatory response.16 Corneal nerves can also become sensitised by local inflammatory mediators such as prostaglandins and bradykinin and thus exhibit spontaneous activity.16 For example, inflammation and/or nerve damage due to trauma, infections and dysregulated metabolism in various ocular conditions, can result in aberrant activation of the sensory nerves of the eye, resulting in neuropathic pain or irritation.34 However, the most common form of corneal hyperalgesia appears to be evaporative dry eye which, in the white eye for example, is presumed to be due to subclinical corneal epithelial inflammation such as appears likely to be triggered by tear hyperosmolarity1 with associated corneal desiccation.9 Noxious stimuli to which the cornea can become hypersensitive include fumes (chemicals) as well as thermal and mechanical sources of irritation.9 Ongoing nociceptor activity can itself cause inflammation which presumably can contribute to hyperalgesia.9 Inflammatory mediators activate changes which lower activity thresholds so that the duration and intensity of responses are escalated.9 Such neuroplastic hypersensitivity explains how a given stimulus can provoke a response which escalates in intensity over time and lasts longer.9 This manifestation of neural plasticity is known as peripheral sensitisation.9 Parra and coauthors concluded from superfused mouse eye studies that tear osmolarity elevations in the range observed in DES predominantly excite cold thermoreceptors, supporting the hypothesis that dryness sensations experienced by these patients are due, at least in part, to an augmented activity of corneal cold thermoreceptors.35 However, inflammation stimulated by hyperosmolarity may increase symptoms by peripheral sensitisation which has developed in response to inflammation provoked by other mechanisms.
Regulation of basal tear production and lacrimationSensory innervation of the cornea is essential for detecting environmental stressors and, through brain stem circuits, for regulating the flow of glandular secretion.18 Glandular secretions from lacrimal, goblet cell, and Meibomian gland sources, are regulated through primary afferent projections to the spinal trigeminal nucleus.18 Conjunctival and corneal epithelial cells can also modify tear composition through the secretion or absorption of electrolytes and water.18 Evidence indicates that basal tear secretion (stimulated innocuously by cold receptors), and secretion evoked by noxious stimulation of corneal nociceptors, are driven by different but possibly overlapping brainstem reflex arcs.18
Apart from dysfunction in aqueous and/or lipid and/or mucin production for example, DES may also be caused by an inability of sensory neurons in the noxious stimulation tear reflex arc to properly regulate tearing.18 Apart from ageing-related reduced corneal sensitivity,36 dry eye symptoms may be accompanied by a decrease in corneal sensitivity such as may be associated with refractive surgery, diabetes.18 herpetic keratitis, topical pharmacological agents and long-term contact lens wear.37 In addition, dry eye-induced alterations to the properties of corneal afferent neurons and the central processing of corneal input may have significant consequences for both the regulation of tearing and ocular pain.18
A longitudinal study of corneal sensitivity in patients with DES found that, notwithstanding highly variable results, corneal sensitivity was found to be lowered in severe dry eye.37 Bourcier and coauthors also found that subjects with dry eye can have reduced mechanical, chemical and corneal thermal sensitivity.26 Similarly, patients over 60 years of age with dry eyes were found to have reduced density of sub-basal nerves but also reduced mechanical, chemical and thermal corneal sensitivity.36 In a study of rats tear hyperosmolarity was found to significantly decrease physiological sensitivity and morphological integrity of the corneal nerves which are important in tear production.38 Similar alterations might contribute to the diminished tearing seen clinically in dry eye patients38 because reduced corneal sensitivity may reduce basal tearing. The degree to which the innocuous stimulation of cold receptors can contribute to basal secretion may also be impaired by reduced corneal sensitivity.
Type II mechanoreceptive neurons in the rabbit cornea were found to respond best to stimuli generated using a nylon filament or human eyelash moving tangentially across the corneal surface.39 This finding suggests that activation of mechanoreceptors in the cornea induces lacrimation and blinking to clear particulate matter from the eye.40 However, mechanoreceptors in the lid wiper may also be involved in stimulating reflex tear production. Each class of primary corneal afferent neuron has the potential to induce tear secretion by responding to different environmental stimuli and, according to the type or combinations of different types of neurons involved, tear composition may vary18 as may the quantity of tears with associated variations in their homeostatic functions. For example, deprivation of afferent neurological stimulation signifies dysfunction of reflex tearing due to a decrease in corneal sensitivity.37 Topical anaesthesia reduces basal tearing, suggesting that background activity in corneal nerve fibres is required to maintain a tonic stimulation of the lacrimal gland.4 By reducing or eliminating this continuous sensory input, it is expected that the reflexively maintained tear flow is reduced.41
Persistent dry eye symptoms following refractive surgeryThe most common complaint by patients who undergo refractive surgery is ocular dryness, which is reported by more than 40% of them, particularly on waking.1 Reduced sensory input as a consequence of transected corneal nerves may help explain dry eye symptoms occurring after refractive surgery.4 However, while it was initially believed that post-laser assisted in situ keratomileusis symptoms were caused by ocular dryness, and referred to as “dry eye” it is now increasingly understood that corneal nerve damage produced by this surgery resembles the pathologic neuroplasticity associated with other forms of persistent post-operative pain.42 In susceptible patients these neuropathological changes, including peripheral and central sensitisation, may underlie certain persistent dry eye symptoms following refractive surgery.42
Psychological factors and exaggerated symptoms including potential influences of mood and sleep disorders, as well as stress and anxiety, on chronic pain and irritationPsychological initiation and modulation of pain perception can involve the influence of emotional and hypnotic suggestion, as well as differences in expectation and anticipation, even in the absence of a physical pain stimulus.43 The perception of pain or irritation can also be modified by mechanisms related to distraction and intensity of attention.43 For example, negative emotional states can enhance the perception of pain and irritation and cognitive modulation of attention can interfere with the perception of pain and irritation.43 Subjective happiness scores were found to be inversely correlated with DE symptoms but patients with symptoms but no signs of a DES had the lowest happiness scores.44
Neuroplasticity can be disrupted by mood disorders.45 Any comprehensive account of the pathophysiology of mood disorders will include consideration of causal roles for psychological and physiological stress.45 For example, the dry eye symptoms of a sample of patients attending a Veterans Affairs Eye Clinic were found to be more closely aligned with non-ocular pain, depression and post-traumatic stress disorder rather than with parameters of dry eye.46 No tear parameters remained significantly associated with dry eye symptoms following multivariable linear regression analysis.46 Chronic stress can precipitate or exacerbate depression as well as disrupt neuroplasticity.45 These responses to chronic stress can be countered by anti-depressant treatment.45 However, anti-depressant medications can contribute to DES.47,48
Dry eye symptoms of pain, dryness, grittiness, itchiness, redness, burning or stinging, foreign body sensation and sensitivity to light and wind can all negatively impact quality of life with a greater risk of depression and anxiety for those with more symptoms.49 All forms of chronic pain are treatable problems affecting an estimated 50% of community-dwelling older adults and having potential consequences of impaired physical function, depression, anxiety, disrupted sleep and appetite, as well as excessive use of health care services.29 A review found that the prevalence of pain of predominantly neuropathic origin in family practices in the United Kingdom was 8%.50 However, the prevalence of pain, discomfort and irritation which includes a neuropathic component may be much higher. For example, neuropathic ocular irritation or pain features can be common in patients with symptomatic dry eye and these features correlate with symptom severity and persistence.28
Assessing neuropathic symptomsAlthough several studies have reviewed how neurosensory dysfunction can be a component of dry eye symptoms in some patients, these aspects of DES are not routinely tested for or treated in the clinical setting.21 Typical testing methods used in clinical practice are inadequate to evaluate the somatosensory status of the cornea for patients with dry eye symptoms which are not directly related to tear dysfunction.51 Consequently, DES patients with symptoms arising from neuropathic mechanisms represent a diagnostic and therapeutic challenge that is more easily dismissed than addressed.9 Confocal microscopy might identify morphological changes in the sub-basal nerve plexus which are related to somatosensory dysfunction.51 Quantitative sensory testing using Cochet-Bonnett or Belmonte aesthesiometers for example, may identify somatosensory dysfunction including both hyper- and hypo-aesthesias (increased and decreased sensitivities).51 Central nervous system sensitisation can be suspected if a local anaesthetic does not provide symptomatic relief.30
Allodynia (pain due to a stimulus which does not usually provoke pain, such as exposure to light or a draught of air or wind) and hyperalgesia (exaggerated pain or irritation response from a stimulus that usually causes pain) are prominent symptoms in patients with neuropathic pain.52 Both types of hypersensitivity are seen in various peripheral neuropathies and central pain disorders, and affect 15–50% of patients with neuropathic pain.52 Although mechanical sensitivity was found to normally decrease with age, increased sensitivity was found in subjects with more severe ocular complaints, wind hyperalgesia and non-ocular pain.51
Nociceptive irritation or pain can be chronic (lasting longer than 3 months) and is related to ongoing injury or insult.9 Chronic and neuropathic have been used interchangeably to describe symptoms but there are mechanistic differences.9 While nociceptive (physiological) pain is related to symptoms which can become chronic and which reflect the degree of injury and resolve when healing is complete, neuropathic pain on the other hand, has the distinguishing feature that it can be chronic in the absence of any obvious injury.9
Assessment of clinical pain or irritation starts with a history of precipitating and exacerbating events, the frequency and duration of symptoms, their character, pattern intensity and radiation, as well as associated symptoms.53 A history of previous management attempts which have not been successful is useful.53 Even lack of efficacy of normal treatments aimed at relief of presumed nociceptive irritation may be an important hint at the presence of a neuropathic component of those symptoms.50 Patients who reported no relief, or only partial symptomatic improvement from using artificial tears to treat dry-eye associated ocular pain, also reported higher levels of hot-burning symptoms and sensitivity to wind when compared to those who reported complete resolution of symptoms.8 The same little or no symptomatic improvement from artificial tear instillation group, also reported higher systemic pain scores.8 Screening tools have been developed to examine for neuropathic pain.53 The McGill Neuropathic Pain Questionnaire consists of 12 sensory descriptors: throbbing, shooting, stabbing, sharp, cramping, gnawing, hot, burning, aching, heavy, tender, splitting and the affective descriptors: tiring, exhausting, sickening, fearful, and punishingly cruel.28
The descriptors of severe neuropathic cornea-projected pain include dry, gravelly, burning, hot, jabbing and aching9 but symptoms of this strength and nature may not necessarily be reported by patients suffering from a DES which is complicated by a neuroplastic mechanism. For example, in a study of primary Sjogren's syndrome patients, alterations in corneal nerve morphology and indications of inflammatory activity suggested that the neuropathic corneal mechanical hypersensitivity found was induced by ocular surface inflammation54 which might have contributed to peripheral sensitisation.1,22,34 The NEI-VFQ-25 vision specific assessment of quality of life takes 10min for patients to complete.55 A dry eye specific quality of life instrument (The Impact of Dry Eye on Everyday Life) consists of 57 questions.55 Even the short form of the McGill Pain Questionnaire which assesses the major symptoms of both neuropathic and non-neuropathic pain requires responses to 22 items.56 The time consuming nature of neuropathic pain and quality of life assessments could limit their application in many clinical settings.
Morphological changes in the corneal sub-basal nerve plexus which are related to somatosensory dysfunction may be detected by confocal microscopy.51 Quantitative sensory testing may identify somatosensory dysfunction including both hyper- and hypo-aesthesias (increased and decreased sensitivities) using Cochet-Bonnett or Belmonte aesthesiometers for example.51 Dry eye symptoms in the absence of concomitant evidence of tear dysfunction and unsatisfactory response to tear dysfunction therapies should prompt consideration of neuropathic mechanisms. Standard treatments for DES do not reduce symptoms of somatosensory dysfunction.51 DES patients with stress and anxiety-related depressive conditions and/or who provide reports of non-ocular pain, may be suspected to have a neuropathic basis for their dry eye symptoms.
Treatment of chronic pain or irritationUnderstanding the neuropathology of dry eye will be important in developing alternative approaches to treating this disorder.21 A multimodal approach may be more beneficial including treating any ongoing ocular surface damage with protective and anti-inflammatory agents, and ocular sensory apparatus dysfunction with anti-neuropathic pain treatment.21 Reducing ocular inflammation may help reduce the risk of any associated neuroplastic peripheral corneal or lid wiper sensitisation. Based on the concept that inflammation is a key component of the pathogenesis of dry eye, a number of anti-inflammatory agents have been used or suggested for use in the treatment of dry eye disease.57 For example, cyclosporine, corticosteroids and tetracyclines57 as well as tumour necrosis factor-α-stimulated gene/protein-6 and prednisolone58 have been evaluated in clinical trials and animal models.
However, awareness of the wide range of potential contributors to ocular irritation and inflammation such as lack of sleep, eyestrain, exposure to environmental irritants such as wind, dust, glare, smoke, smog and allergens, provides scope for behaviour changes which reduce ocular inflammation. In addition, the ocular surface may become inflamed in response to preservatives in contact lens solutions, tear supplements or other therapeutic products and help to initiate or maintain inflammation. Given its biochemical properties, autologous serum is a therapy of interest in treating corneal somatosensory pathway dysfunction in dry eye, including the presence of various neuromediators (including nerve growth factor) which may affect corneal nerve function.59 In addition, autologous serum suppresses apoptosis in corneal and conjunctival epithelium60 and may be of benefit in treating ocular surface epitheliopathy related to DES. A recent review of therapeutic strategies for treating dry eye in ageing populations describes a range of other alternatives.23
Persistent ocular symptoms may contribute to the development or exacerbation of stress, anxiety, depression and sleep disorders.50 Conversely, psychosocial problems associated with stress, anxiety, depression and sleep disorders may contribute to the initiation or exacerbation of neuropathic processes and early detection of them is of special importance in management.50 Neurological dysfunction can be classified according to whether it is influenced by the hypothalamus and the limbic system, the decrease of afferent neurological stimulation, or the deprivation of efferent neurological stimulation.41 Hypothalamic and limbic influences can be due to stress, anxiety, or psychological imbalances, which may interfere with the physiological circadian rhythm of basal tear production.37 For example, management of stress due to psychosocial causes should be an integral part of treatment plans50 for cases of neuropathic pain.
First-line medications for neuropathic pain include tricyclic antidepressants, anticonvulsants, opioids and opioid-like drugs. 29 The Gate Control Theory emphasizes the central role of the brain in pain processing which accounts for the success of cognitive behavioural therapy, especially for depression.29
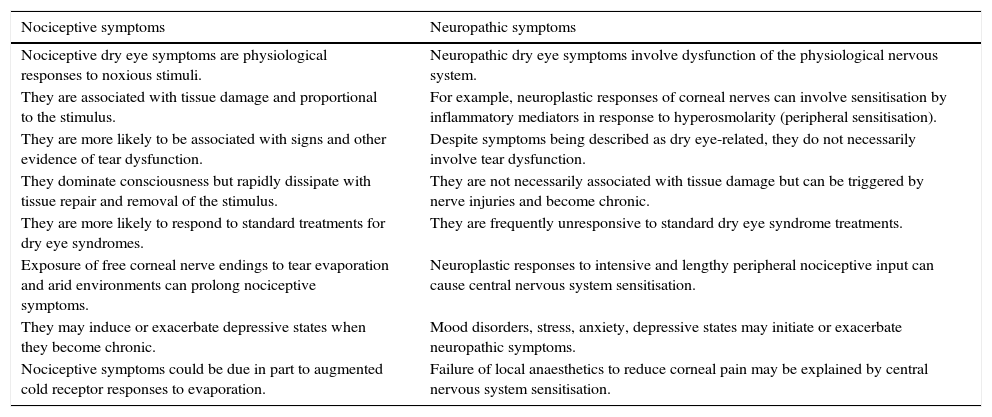
DiscussionNociceptive symptoms are the normal physiological responses to noxious stimuli associated with tear dysfunction. Management of DES may be complicated by neuropathic symptoms and failure to consider their involvement may undermine treatment outcomes. Table 1 describes some of the characteristics for these two symptom classes. Neuropathic ocular pain due to dry eye can be associated with multiple comorbid chronic pain syndromes.61 Although neuropathic contributions to DES are more likely to be associated with more severe and/or chronic symptoms, it is not known to what extent mild to moderate DES symptoms can include a neuropathic component. For example, symptoms which are disproportionate to the signs of dry eye disease could indicate that they have a neuropathic basis. Lack of a satisfactory symptomatic response to a treatment such as regular use of artificial tears may be a consequence of failure to diagnose and treat Meibomian gland dysfunction for example, but could also indicate a neuropathic contribution to symptoms. Psychosocial problems associated with stress, anxiety, depression and sleep disorders may contribute to the initiation or exacerbation of neuropathic processes.50 Inflammation and/or nerve damage due to surgery can result in aberrant activation of the sensory nerves of the eye, resulting in neuropathic pain or irritation.34 Reducing ocular inflammation may help limit neuropathic symptoms.
Some distinctions between nociceptive and neuropathic symptoms, which may present separately but can co-exist.
| Nociceptive symptoms | Neuropathic symptoms |
|---|---|
| Nociceptive dry eye symptoms are physiological responses to noxious stimuli. | Neuropathic dry eye symptoms involve dysfunction of the physiological nervous system. |
| They are associated with tissue damage and proportional to the stimulus. | For example, neuroplastic responses of corneal nerves can involve sensitisation by inflammatory mediators in response to hyperosmolarity (peripheral sensitisation). |
| They are more likely to be associated with signs and other evidence of tear dysfunction. | Despite symptoms being described as dry eye-related, they do not necessarily involve tear dysfunction. |
| They dominate consciousness but rapidly dissipate with tissue repair and removal of the stimulus. | They are not necessarily associated with tissue damage but can be triggered by nerve injuries and become chronic. |
| They are more likely to respond to standard treatments for dry eye syndromes. | They are frequently unresponsive to standard dry eye syndrome treatments. |
| Exposure of free corneal nerve endings to tear evaporation and arid environments can prolong nociceptive symptoms. | Neuroplastic responses to intensive and lengthy peripheral nociceptive input can cause central nervous system sensitisation. |
| They may induce or exacerbate depressive states when they become chronic. | Mood disorders, stress, anxiety, depressive states may initiate or exacerbate neuropathic symptoms. |
| Nociceptive symptoms could be due in part to augmented cold receptor responses to evaporation. | Failure of local anaesthetics to reduce corneal pain may be explained by central nervous system sensitisation. |
The author has no conflicts of interest to declare.