Epidemic keratoconjunctivitis (EKC) is an ocular surface infection caused by adenovirus. To date, there are no approved topical antiadenoviral therapeutics to treat EKC. Recent research reveals that treatment with topical corticosteroids for symptomatic relief of EKC enhances adenovirus replication and delays cell shedding from the ocular surface which delays adenovirus elimination. The current management of EKC largely revolves around accurate diagnosis of the condition and implementation of disinfection protocol to prevent its spread. Development of an effective antiviral treatment that addresses inflammation and does not prolong viral shedding would provide significant benefit. The literature reports on a variety of therapeutics that could potentially satisfy this deficiency. Topical ganciclovir and povidone-iodine combination drops have shown the most recent potential, but both therapeutics need to be investigated in larger scale studies. Until an antiadenoviral option is produced, the treatment of EKC should maintain a judicious case by case approach aiming to contain its dissemination and prevent visual consequences.
La queratoconjuntivitis epidémica (QCE) consiste en una infección de la superficie ocular causada por adenovirus. Hasta la fecha no existen terapias antiadenovíricas aprobadas para tratar la QCE. Las investigaciones recientes revelan que el tratamiento con corticoesteroides tópicos para un alivio sintomático de la QCE aumenta la replicación del adenovirus y retrasa la descamación de células en la superficie ocular, retardando la eliminación del virus. El tratamiento actual de la QCE gira alrededor del diagnóstico preciso sobre la condición clínica y la introducción de un protocolo de desinfección que impida su diseminación. El desarrollo de un tratamiento antivírico eficaz que trate la inflamación y no retrase la eliminación vírica supondría un beneficio considerable. La literatura reporta gran variedad de terapias que podrían satisfacer potencialmente esta deficiencia. La aplicación de gotas tópicas con una combinación de ganciclovir y iodina-povidona ha demostrado recientemente su potencial, aunque ambas terapias precisan investigarse mediante estudios a gran escala. Hasta que aparezca una opción antiadenovírica el tratamiento de la QCE debería realizarse utilizando un enfoque caso a caso, a fin de contener su diseminación y prevenir consecuencias visuales.
Epidemic keratoconjunctivitis (EKC) is caused by the adenovirus pathogen.1,2 Adenoviral conjunctivitis is known to be the most common cause of red eye in the world.3 A study at the Wills Eye Hospital emergency room found a 62% prevalence of adenoviral conjunctivitis amongst all patients presenting with a clinical diagnosis of infectious conjunctivitis, while various other studies have demonstrated a prevalence of between 15% and 70% of all conjunctivitis worldwide.1,3 More than 50 different adenovirus serotypes have been identified and divided into six distinct subgroups.2,4,5 Specific adenovirus serotypes are associated with various types of ocular infection.1,2 The most common types of adenoviral conjunctivitis include EKC, pharyngoconjunctival fever and nonspecific follicular conjunctivitis (simple adenoviral conjunctivitis).1,6 EKC is commonly associated with adenovirus serotypes 8, 19 and 37.2,4,6–8 EKC is considered a more critical form of adenoviral keratoconjunctivitis because of the adverse consequences it may have on visual acuity.8
Due to the epidemic nature of EKC, outbreaks have been studied in hospitals and health-care settings.9–11 EKC may occur in crowded living conditions and places where people come into contact with one another, such as schools and medical practices.1,9 Transmission of the virus occurs by direct contact through ocular and respiratory secretions or by indirect contact with contaminated instruments or solutions.11 Viral particles have been shown to be infectious on nonporous surfaces for upwards of one month and there is evidence that transmission can occur through inanimate vectors, such as door handles and tonometer heads.4,9
The dilemma with a virus such as EKC is that patients who have contracted the disease are asymptomatic during the incubation period and may inadvertently spread the virus.9 This is how eye doctors may unknowingly act as vectors and contribute to the spread of EKC from patient to patient.9 The current literature demonstrates that evidence-based medicine has yet to proffer a sufficient therapeutic method to treat EKC.5 The fact remains that EKC is highly contagious.9,12
DiscussionEKC is an ocular surface infection associated with a marked inflammatory reaction, and symptoms of redness, irritation, tearing, blurry vision and sensitivity to light.4 Clinical signs include eyelid edema, follicular conjunctivitis, conjunctival edema and hyperemia, epithelial keratitis and often preauricular lymphadenopathy.6 The onset of EKC may seem rapid to the patient, but in reality, there is an incubation period of about one week before the clinical symptoms present.12 The second eye is often affected days later to a much lesser degree.13 The period of communicability is from late in the incubation phase up to 14 days after the onset of the disease.14 This acute phase of EKC is marked by a severe conjunctivitis and lasts from two to four weeks.14 After the conjunctivitis appears, there is a period of viral shedding where the self-limiting virus is gradually cleared from its host.12 However, before the virus is completely shed, the inflammatory reaction of the conjunctiva can become so intense that it results in a pseudomembrane and potentially permanent symblepharon formation or punctual occlusion.6,12
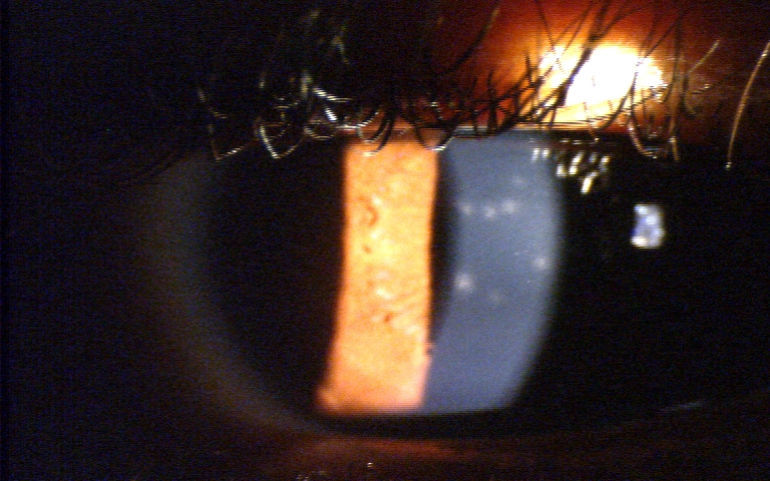
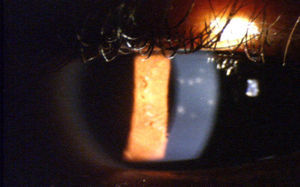
The symptoms and duration of EKC can vary widely; however, it is distinguished from other adenoviral infections when the cornea becomes affected by the development of multifocal subepithelial infiltrates (SEIs)6 (see Fig. 1). The hallmark of the second, more chronic phase of EKC is the corneal involvement.14 The patient's presentation of multifocal subepithelial corneal infiltrates (SEI) supports the diagnosis of EKC, as the presence of SEIs are considered pathognomonic for the diagnosis of EKC.6 These infiltrates are typically observed within seven to ten days after the onset of the initial signs of infection.6 It is possible for the infiltrates to persist for extended periods of time ranging from months to years, and their presence can also cause reduced visual acuity.4,12 A study by Butt and Chodosh found that 25.9% of patients demonstrated chronic corneal inflammation with symptomatic SEIs present for more than 45 days from the first examination.4
Accurate diagnosis of EKC upon presentation is essential.1,12 Misdiagnosis can lead to inaccurate patient expectations for resolution, as well as, a lack of patient education on appropriate disinfection precautions. Preventing the spread of EKC may be the most proactive way of treating it.1 This largely depends on the eye care practitioner making the correct diagnosis and instituting the appropriate treatment before the patient leaves the provider's office.1,12 The patient should be aware that EKC is easily transmitted by contact with an inert surface, such as a door handle, and to avoid rubbing eyes and then touching anything.9 Frequent hand washing should be implemented and infected patients should not share towels or cosmetics.15 Contact lens wearers should be told to throw away their disposable lenses and use a new pair only after the infection has cleared.15 The patient should be educated that the contagious period for EKC may last upward of 14 days from when the symptoms began and caution should be exercised if returning to school or work before that time.13 Kaufman recommends that health care providers who contract adenoviral conjunctivitis spend two weeks isolated from patient care in order to prevent the spread of the disease.12
Due to its highly contagious nature, an outbreak of EKC in a doctor's office can have a snowball effect, quickly spreading from one patient to the next.12 A proper diagnosis is necessary to implement extra precautions with disinfection procedures and decrease patient morbidity.3,12Table 1 highlights basic protection control procedures that should be implemented on a regular basis to prevent any disease transmission, including EKC, in the medical provider's office.16 When available, single-use disposable devices should be employed to assist in the examination of the patient.15 This includes disposable medical gloves, disposable droppers, cotton-tipped applicators and disposable tonometer prism or shields that can all be discarded immediately after use.15 A tonopen with a sterile, disposable tip cover would be a practical tool to use upon presentation of any potentially contagious conjunctivitis patient.15 In the case of suspected or known presence of adenovirus, emphasis can also be placed on proper disinfection of the exam room and any instruments that came into contact with the patient.15 Lakkis et al. recommend cleaning all large surfaces in an optometric practice with isopropyl alcohol tissues, 30% alcohol solution or sodium hypochlorite solution (obtained with a 1:5 dilution of 5% household bleach).15 With the increasingly common use of electronic health records in today's practice, it is also recommended to wipe computer keyboards with alcohol each day.15
Universal protection control procedures.16
| (1) Employ proper hand washing technique before and after every patient. |
| (2) Use disposable medical gloves as a barrier precaution when in contact with any potentially infectious material. |
| (3) Use gowns and masks as barrier precautions with the possibility of blood splatter, and masks if an airborne pathogen is suspected. |
| (4) Protective eyewear is recommended when contaminated fluids or blood could splash into the examiner's eyes. |
| (5) Minimize contact with infected tissue through “no touch” techniques such as finger cots, cotton-tipped applicators or other instrumentation. |
| (6) Dispose of sharps instruments in appropriate waste containers. |
| (7) Instrument disinfection is necessary for all instruments that come in contact with the ocular adnexa, including the cornea and tears. |
| (8) Instrument sterilization is necessary for all instruments that come into contact with the vascular system. |
| (9) Follow contact lens disinfection procedures recommended by the CDC. |
| (10) Dispose of infectious waste according to federal, state and local guidelines. |
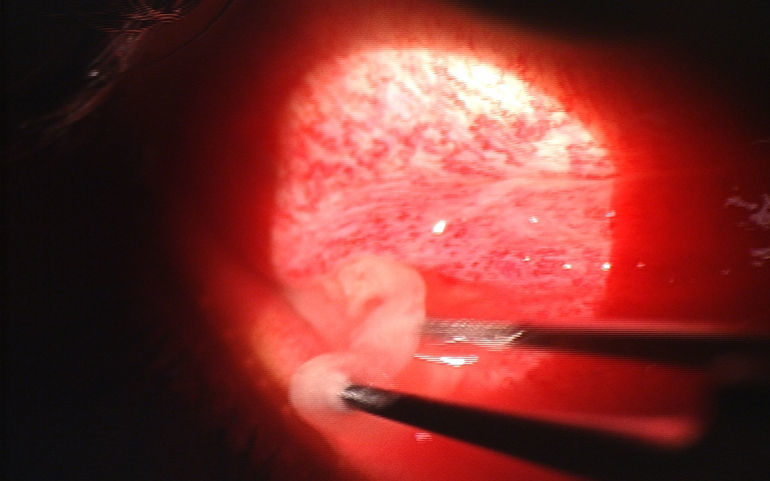
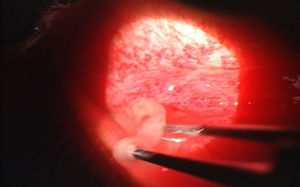
There are no universal guidelines for optometrists in regard to decontamination of ophthalmic instruments.15 The Centers for Disease Control (CDC) has published an extensive document entitled Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 which describes evidence-based recommendations for cleaning, disinfection and sterilization of medical devices.17 Maximum effectiveness for infection control is dependent on cleaning as the initial step.17 Cleaning is defined as removing all visible organic and inorganic material from an object or surface.17 Instruments that have come in contact with mucous membrane tissue or non-intact skin are classified by the CDC as “semicritical items” which at minimum require high-level disinfection using chemical disinfectants.17 The CDC recommends that tonometer tips are wiped clean and disinfected for 5–10min with either 3% hydrogen peroxide, 5000ppm chlorine, 70% ethyl alcohol or 70% isopropyl alcohol, then thoroughly rinsed in tap water and air dried before use.17 The CDC guidelines document recognizes that there is some evidence that 3% hydrogen peroxide and 70% isopropyl alcohol may not be effective against EKC-causing adenovirus.17 In contrast, one study showed that adenovirus 8 is effectively eliminated from tonometer tips with a disinfectant wipe containing isopropyl alcohol, hydrogen peroxide or iodopher.18 The same study also demonstrated that no virus was recovered from Goldmann tonometer tips following a 5-min soak in the same disinfectants that composed the wipes.18 This contradiction may exist secondary to the studies being performed in a controlled laboratory setting and further studies are needed for a clear recommendation.17 The FDA requests the manufacturers of medical devices to include at least one validated cleaning and disinfection or sterilization protocol in the labeling of their devices.17 Therefore, it has been suggested to refer to the manufacturer's recommendations for protocol on disinfection or sterilization of ophthalmic instruments.15 Haag Streit details a step-by-step process for disinfecting tonometer tips on its website and Volk Optical Inc. has a very extensive “Cleaning & Care Guide” on its website that provides cleaning, disinfection and sterilization methods for its various lenses.19,20 The CDC classifies “critical items” as those that confer a high risk for infection such as objects that enter sterile tissue or the vascular system.17 These objects must undergo sterilization which is accomplished with steam, or heat-sensitive objects may be treated with approved chemical sterilants.17 Sterilization is recommended for any instruments that may have come in contact with blood, as is possible with pseudomembrane removal in an EKC patient17 (see Fig. 2). These instruments should be properly cleaned and disinfected first, then wrapped in peel pouches and sterilized by the eye care provider in office using a tabletop steam autoclave unit.16
The clinical diagnosis of an adenovirus infection is typically made based on the history and presenting signs and symptoms.3 In reality, it may initially be difficult to clinically distinguish some viral conjunctivitis from bacterial conjunctivitis.1,3 The traditional gold standard for diagnosis of EKC or any adenovirus conjunctivitis has been cell culture in combination with immunofluorescence staining (CC-IFA).1,3 Other laboratory diagnostic methods used to identify adenoviral infections include serologic methods, antigen detection and polymerase chain reaction (PCR).1,3 Some consider PCR the new gold standard for diagnosis because it is very specific and has proven to be more sensitive than CC-IFA.1,3 To date these methods are the most accurate way to confirm the cause of conjunctivitis; however, many doctors’ offices lack direct access to diagnostic lab procedures.1 Both the prohibitive cost and time delay in referring a patient out for laboratory diagnostic testing and then waiting to receive the outcome, present a diagnostic challenge to physicians.3 The result of this dilemma is that diagnostic and treatment decisions are made based upon presenting signs and symptoms, along with previous clinical experience.3 Thus, a disease with a presumed etiology may risk being inadvertently mistreated.
There is recognition of the potential benefit of a rapid accurate diagnostic test to help identify patients presenting with adenoviral infection.9 Cheung's study found that standard viral culture techniques are not satisfactory due to the time it takes for the viral culture swabs to produce a result.9 Rapid diagnosis of infection has improved with non-culture-based techniques.5 Sambursky et al. report the introduction of the Rapid Pathogen Screening (RPS) Adeno Detector which is described as “a rapid point-of-care diagnostic test for the visual, qualitative in vitro detection of adenoviral antigens directly from human eye fluid.”3 Clinical research suggests that a sensitivity of 88% and specificity of 91% in comparison to CC-IFA makes the RPS Adeno Detector useful for in office detection of adenoviral conjunctivitis.3 The RPS Adeno Detector works with a direct sample and no extraction or dilution step is required.3 A “Point of Care” device such as the RPS Adeno Detector with immediate and easy-to-read results may prove useful in determining proper diagnosis of in-office patients whose cases are not already evident.21
Despite the fact that EKC is a self-limiting disease, most affected individuals seek and receive treatment as a result of the severity of their symptoms.7 The treatment of EKC often includes palliative treatment, such as cool compresses and artificial tears.6 In various instances, EKC treatment has also consisted of topical antibiotics, topical nonsteroidal anti-inflammatory drugs (NSAIDS) and topical corticosteroids.4,6 Supportive therapy is an obvious choice for treatment because there are currently no approved, effective anti-adenoviral therapies.5,7,8 However, one study showed that 36% of eye care practitioners surveyed prescribed topical corticosteroids to treat EKC.22 Most clinicians agree that clinical use of topical corticosteroids is justified in cases of acute EKC where there is the threat of incapacitating visual loss from persistent subepithelial infiltrates, pseudomembranous conjunctivitis and iridocyclitis.4,22–24 Steroids suppress conjunctival and corneal inflammation to provide symptomatic relief, but they do not act to shorten the course of EKC.25
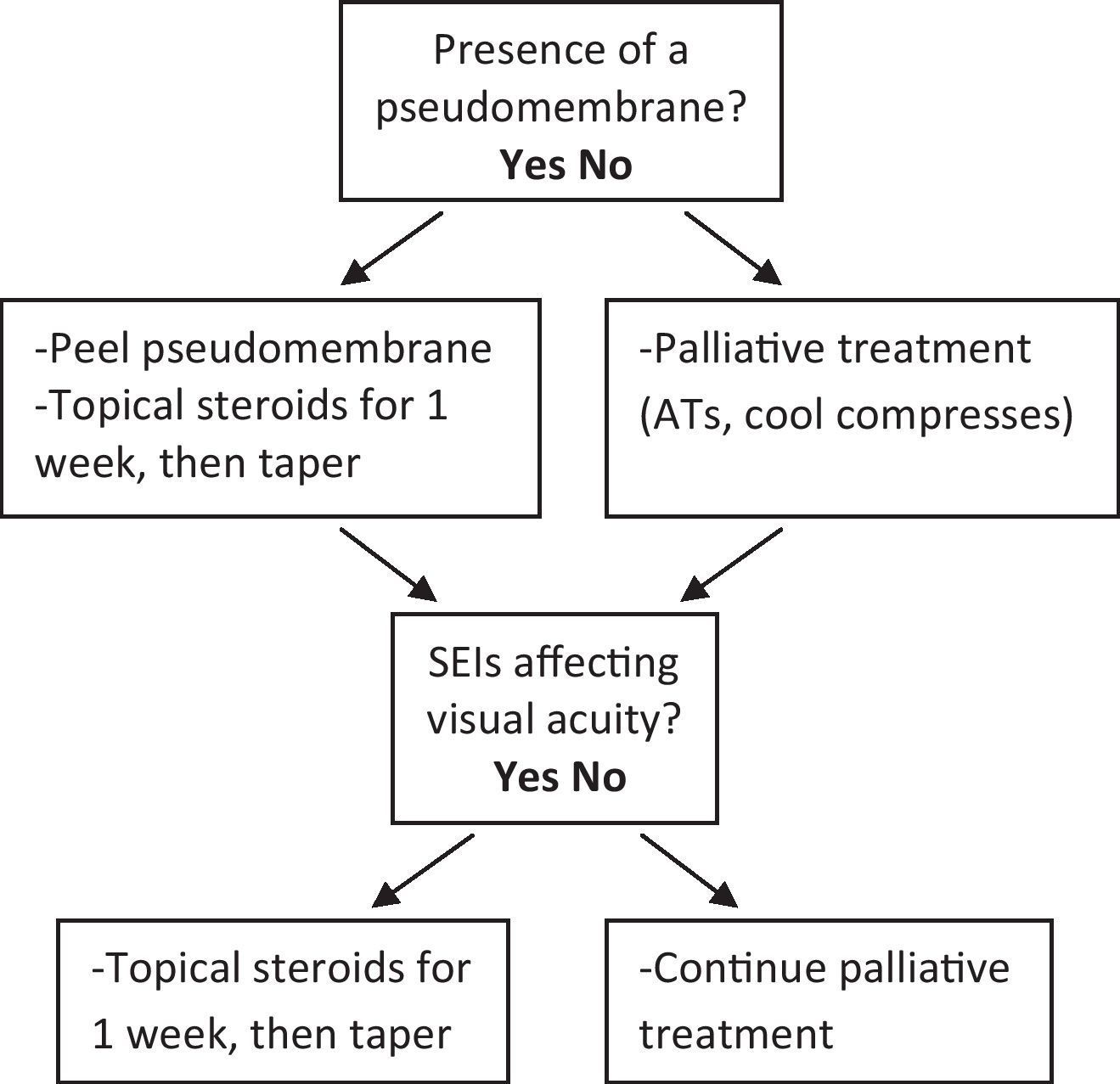
It is important to recognize the risks and indications for proceeding with corticosteroids when treating presumed ocular viral infections. The possibility of misdiagnosing an early presentation of herpes simplex type I, acanthamoeba or fungal infection in combination with use of topical corticosteroids could be detrimental to a patient's ocular health.23,25 The increased incidence of side effects from long-term use such as cataracts and glaucoma should be considered, but are less likely to be a factor when treating acute disease.23 When corticosteroids are used, they are often reserved until patients present with a pseudomembrane or symptomatic subepithelial infiltrates4,26 (see Table 2). However, there is clinical evidence that this prolongs the duration of SEIs.4 The literature also reports that both long- and short-term use of topical corticosteroids enhances adenovirus replication and prolongs adenovirus shedding.23,25 Researchers have suggested that normal adenovirus clearance is inhibited by the strong anti-inflammatory and anti-immune effects of the corticosteroid.23 Romanowski et al. demonstrated in numerous experimental studies that corticosteroids, ranging from the more potent 1% prednisolone acetate to the less potent 0.12% prednisolone acetate, all increased the duration of viral shedding in varying degrees.23,25 One of these studies found that long-term treatment with 1% prednisolone acetate profoundly altered immune clearance and could potentially increase the risk of transmission and promote the number and extent of community and medical facility epidemics.25 Another concluded that the risk of using a corticosteroid and prolonging an infection must be weighed against the clinical benefit of short-term more limited potency corticosteroids used for symptomatic relief.23 The controversial use of corticosteroids in conjunction with EKC treatment would not need to be a consideration with the development of an anti-inflammatory therapeutic that does not inhibit adenoviral clearance or an effective antiadenoviral agent.
Adenoviral conjunctivitis treatment recommendations.26
The effects of non-steroidal topical therapeutics on adenovirus conjunctivitis have been explored. NSAIDS do not affect adenovirus clearance but have demonstrated no better relief to patient symptoms from viral conjunctivitis than artificial tears.27,28 Cyclosporine was specifically explored for treating symptoms caused by subepithelial infiltrates.29,30 Though one study found subjective improvement of local symptoms with 1% cyclosporine eyedrops, as compared to 0.2% cidofovir or a combination of 0.2% cidofovir+1% cyclosporine; no acceleration in improvement of clinical symptoms was found compared to the natural course of the EKC infection.29 Romanowski et al. found that treatment with both 2.0% and 0.5% cyclosporine A in the rabbit model significantly reduced the formation of SEI's.30 However, use of topical cyclosporine A increased adenoviral replication, similar to the results reported with corticosteroid use.30
Research has been directed toward the treatment of adenoviral keratoconjunctivitis with antiviral compounds. Ribavirin has been explored in clinical studies and was found to have restricted in vitro activity and lack efficacy against the three predominant adenovirus serotypes associated with EKC (Ad8, Ad19 and Ad37).7,8 Nucleoside analogues are also known to be active against adenoviral infections and examples of this drug class include cidofovir and ganciclovir.31 Success in cidofovir studies using the rabbit model led to clinical trials in the USA to investigate cidofovir against human ocular adenovirus.8 An early study by Hillenkamp showed no acceleration in improvement of EKC clinical symptoms with 0.2% cidofovir; however the authors speculated that the concentration of cidofovir was too low.29 Another study evaluating the efficacy of cidofovir 1% ophthalmic preparation with and without cyclosporine A 1% demonstrated that cidofovir decreases the frequency of severe corneal opacities.32 However, its clinical use was deemed limited because the frequent administration was associated with local toxicity.32 Cidofovir development has been stopped in the USA secondary to clinical concerns about its toxicity and because of evidence regarding possible emergence of clinical resistance.8
Topical ganciclovir 0.15% ophthalmic gel is now available commercially in the United States. With topical administration, ganciclovir 0.15% ophthalmic gel achieves therapeutic levels in the aqueous and cornea and is a known effective treatment for herpetic epithelial keratitis.33 Ganciclovir has been advocated for ‘off-label’ use against EKC because it has shown potential against specific adenovirus serotypes in vitro.8 A study in Saudi Arabia compared the effects of ganciclovir 0.15% ophthalmic ointment with preservative-free artificial tears for 18 patients with adenovirus keratoconjunctivitis.34 The ganciclovir group demonstrated resolution of the conjunctivitis in 7.7 days, as opposed to 18.5 days for the artificial tears patients.34 Additionally, only two of the cases treated with ganciclovir developed subepithelial opacities while their presence was detected in seven cases treated with artificial tears.34 The authors of this study determined that topical ganciclovir 0.15% ophthalmic ointment is safe and effective for the treatment of adenoviral conjunctivitis.34 The effects of ganciclovir on adenoviral keratoconjunctivitis need to be demonstrated on a larger scale as well as show its effectivity against adenoviral strains seen in other countries.12
Promising results have been noted in the treatment of adenovirus conjunctivitis with povidone-iodine (PVP-I). Clinical practice has seen the use of ocular applications of povidone-iodine in various capacities including pre- and postoperative ocular surgical prophylaxis, prevention of neonatal conjunctivitis and treatment for bacterial conjunctivitis.35–37 Povidone-iodine has a broad antimicrobial spectrum in vitro and effectively kills bacteria, fungi, viruses and protozoa.35,36 A study by Pelletier et al. investigated a topical combination agent that contained 0.4% povidone-iodine as an anti-viral and 0.1% dexamethasone as a potent steroid.37 The expectation of the study was to “effectively treat both the inflammatory and infectious components of acute adenoviral conjunctivitis by decreasing the symptomatic period following infection, shortening the duration of viral shedding and reducing the potential for infectious transmission.”37 The study followed the clinical course of positive RPS Adeno Detector conjunctivitis while monitoring serial virology markers by quantitative PCR and CC-IFA.37 The results demonstrated that all eyes had rapid improvement of conjunctival injection and discharge with a decrease in viral titers at the same time, warranting a need for further study of this combination agent.37 The authors of another study confirmed “the efficacy of topical dexamethasone 0.1%/povidone-iodine 0.4% (FST-100) in reducing clinical symptoms and infectious viral titers in a rabbit model of adenoviral keratoconjunctivitis.”38 This study concluded that FST-100 has the potential to become the preferred drug in treating adenoviral conjunctivitis in humans and could be most effective in the treatment of more serious forms of infection, including EKC.38 Reports of off-label use of povidone-iodine have also claimed to preserve visual function and reduce viral spread.39 One protocol calls for the medical provider to anesthetize the affected eye, instill a pretreatment NSAID, then instill four to five drops of 5% povidone-iodine solution, have the patient roll his or her eyes around for full exposure, swab the lid margins with the solution while the patient waits for approximately 60s and finally lavage the ocular tissue with sterile saline irrigating solution.39 Development continues of various compounds that have demonstrated anti-adenoviral activity. The endogenous microbicide agent N-chlorotaurine has shown evidence of antimicrobial activity against EKC and was also well-tolerated with minimal side effects.40,41 There is clearly a need for a potent anti-viral drug that addresses patient symptoms, prevents SEI formation and does not interfere with viral clearance.30
ConclusionUntil an effective antiviral or a better alternative to treat EKC becomes available, clinicians must be cautious in their use of corticosteroids. Eye care providers must carefully consider the risks involved with the use of a corticosteroid for symptomatic relief of EKC and its potential to prolong an infection. The extended period of adenovirus shedding associated with the use of corticosteroids could also enhance the spread of EKC and lead to increased local epidemics. Even with the current limited treatment options for EKC, the fact still remains that intervention is required during the viral replication stage, and patients often do not solicit medical consultation until the inflammatory manifestations interfere with their daily activities. Eye care providers should be aware that their management decisions in treating adenoviral conjunctivitis play a very important role in containing epidemics and minimizing patient morbidity. While the search for anti-viral therapy with better efficacy and minimal side-effects continues, the management of EKC will continue to present a clinical dilemma. Accurate diagnosis and prevention of transmission should be stressed as the first line of defense available to contain the dissemination of EKC. The current therapeutic treatment regimen for an EKC patient's signs and symptoms needs to maintain a judicious case-by-case approach to prevent any visual consequence(s).
Conflicts of interestThe authors have no conflicts of interest to declare.