This study aims to estimate the efficacy and safety of a new intense pulsed light (IPL) Thermaeye Plus for dry eye disease (DED) secondary to meibomian gland dysfunction (MGD).
Patients and methodsThis is a prospective, longitudinal study of patients with moderate to severe MGD. Treatment consisted of 4 consecutive IPL sessions, 12 flashes with the same energy 8J/cm2 on the periocular area at days 1, 14, 28, and 49. All patients were evaluated before each IPL sessions and 1 and 4 months after the last session. Measuring: Symptoms score and OSDI questionnaire, Non-invasive tear meniscus height (NITMH), Non-invasive break up (NITBUT), Tear osmolarity (TO), Corneal fluorescein staining (CFS) and Eyelid margin and Meibomian gland assessment. The adverse effects on the eye, periocular area, and systemic complications were evaluated. All types of skin pigmentation (Fitzpatrick scale I to VI) were included.
ResultsThe study included 44 consecutive patients (88 eyes), 40 males and 48 females, with a mean age of 52.5±13.6 years, ranging from 22 to 78 years. Significant improvements were observed in single and total signs and symptoms. The most significant changes were observed in dryness, foreign body sensation (p<0.001), and pain (p<0.005). The OSDI questionnaire showed a significant decrease in total symptoms (p<0.003). The percentage of patients with a normal index <13, improved from 23.8% (10 patients) at baseline to 80.9% (34 patients) at last visit, after 23 weeks. Clinical sings also improved, more than 90%, stand out telangiectasia and blepharitis (p<0.002, and p<0.0005, respectively). No statistical differences between age (<40y, 40–60y, and >60y) and gender were observed. The clinical improvement began after the second and third week after the first IPL session, until the end of the IPL treatment (D49). After that, the results maintained stable until the last visit, after 11 weeks. No complications were reported locally, nor in the periocular area as well as systemically.
ConclusionThermaeye Plus, a new IPL for ophthalmic use, is a safe and effective treatment in patients with DED due to MGD, showing improvement from the second week of treatment and maintaining until the last visit, 4 months after the last IPL session (23 weeks of total follow-up). Patients with pigmented skin (Fitzpatrick V or VI) have been treated with no side effects. It is relevant, especially to apply in brown-dark skin populations since complications with other IPL devices have been reported.
Meibomian gland dysfunction (MGD) is a common condition associated with Dry eye disease (DED), representing the leading cause of the evaporative component. MGD increases the DED severity with a clinically adverse impact on patient's life quality.1 The prevalence of MGD varies geographically and by age, with reports of 5–20% in Western countries to 45–70% in Asiatic.2
The pathogenesis of MGD and DED has been described as a vicious cycle in which inflammation is the essential factor.2 MGD is triggered by different factors (skin disorders, eyelid inflammation, and microbial infections), resulting in ductal epithelium hyperkeratinization and increased meibum viscosity. The glandular ductus is filled with degenerate and viscous meibum, which contains keratinized cellular material accumulating inside the glands (intraglandular cystic dilatation), resulting in its atrophy.3,4 Consequently, the lower outflow of the meibum induces the proliferation of commensal bacteria,5 releasing fatty acids and mono and diglycerides into the tear film, provoking more inflammation and irritation of the ocular surface.6 The result is the instability of the tear film exposing the corneal surface, with the involvement of the subepithelial nerve plexus and more inflammation7 triggering the vicious DED cycle that would feedback the MGD cycle.
Different treatments have been proposed; the use of preservative-free artificial tears, anti-inflammatories, and cyclosporine; antibiotics to control the ocular surface microbiota or the presence of Demodex; dietary supplements with Omega-3 fatty acids to improve the lipid composition of the meibomian glands and tear film; warm compresses, and lid hygiene; radiofrequency to decrease the viscosity of the meibum, mechanical expression of the Meibomian glands, and intraductal probing.8 The truth is that no one achieves a fully effective long-term improvement. Intense pulsed light (IPL) can successfully treat DED symptoms and signs by decrease in symptoms due to MGD, tear film abnormality, and tear inflammatory markers.9
The exact IPL mechanism is unknown. At present, various effects have been proposed trying to explain this technology. The most important are, (1) warming the meibomian glands to facilitate the expression and release of the meibum inside10; (2) improving the function of the Meibomian glands11; (3) inducing intravascular thrombosis of the small blood vessels surrounding the Meibomian glands and telangiectasia of the eyelid margin, reducing the levels of proinflammatory mediators that contribute to dry eye.9,12 (4) reducing tear osmolarity, helping to control the inflammation of the ocular surface, which is one of the key factors in dry eye pathogenesis.12–14 (5) reducing the load of Demodex mites that stimulate secondary inflammation15; (6) improving cellular functions such as collagen synthesis, fibroblasts regeneration and motility in immunoregulatory cells16,17; (7) inducing a neurotrophic effect on the cornea and ocular surface and a neuroimmunomodulatory effect on the meibomian glands.18,19
Many patients suffer from an evaporative dry eye secondary to MGD, with limited studies showing the benefits of IPL for its treatment. Therefore, we evaluated the effects of a new IPL platform, Therameye Plus, in the treatment of evaporative dry eye secondary to MGD and with the advantage that it can be applied to all skin types on the Fitzpatrick scale, up to grade VI.
Material and methodsThe study is prospective, longitudinal, and open-label. All patients were treated with Intense Pulsed Light (IPL), Thermaeye Plus, with four consecutive sessions at days: 1 (D1), 14 (D14), 28 (D28), and 49 days (D49), with a follow-up of minimum 4 months after the last IPL session. Participants were assessed and treated at a single site. This study was conducted according to the tenets of the Declaration of Helsinki and the ethical approval of our Institution. Subjects were recruited from the Department of Ophthalmology of the Hospital Universitari Dexeus, Universidad Politecnica de Catalunya (Barcelona, Spain) between September 2018 to February 2019. A signed informed consent form was obtained from all participants before enrolment.
The inclusion criteria were healthy adult patients, between 20 to 80 years that accept and sign the informed consent and manifest the intention to follow the protocol indicated in the informed consent, attend all visits, and satisfy the inclusion and exclusion criteria. Inclusion criteria consisted of the chief complaint of two of the following symptoms: dryness, foreign body sensation, burning and tearing for more than 3 months and a current diagnosis of DED, and MGD in both eyes. The diagnosis of DED and MGD was defined with the following criteria: (I) Patients with two or more of the following signs in both eyes: redness or thickening of the lid margin, telangiectasia of the lid margin, reduced or no secretions, poor quality secretions and gland capping. (II) Presence of three or more of the following conditions: tear break-up time (NITBUT) <5s. Meibomian gland score (MGS) using the abbreviated MGD grading system for clinical trials, >10; corneal fluorescein staining (CS) using the Baylor grading schema, >10; subjective symptom score (using the OSDI questionnaire), >13; and 5) a tear film osmolarity (TO) ≥310mOsm/L, or a TO difference between both eyes ≥8mOsm/L. Schirmer 1 test (S1 T), 5min without anesthesia, <5mm. No patient was excluded due to the degree of skin pigmentation, all grades were included, according to the Fitzpatrick scale from grade I, to the more pigmented skin, grade VI.
The exclusion criteria included patients with a history of ocular trauma, severe ocular surface disease, active allergy or inflammatory disease at the ocular surface unrelated to DED or MGD; uncontrolled systemic disease or use of systemic medications altering the tear film or affecting the ocular surface; use of any drops other than artificial tears within the last month; recent or current treatments for DED or MGD as radiofrequency or IPL; skin treatments within the last month; current use of punctual plugs; probing or mechanical expression of the meibomian glands in the previous month; lacrimal drainage disease; pigmented lesion in the treatment area. Patients who presented dry eye after corneal refractive surgery (PRK and LASIK) or patients wearing contact lenses were also excluded.
Treatment procedureThe subjects received four separate treatment sessions, the first day (D1), after 14 days (D14), after 28 days (D28), and after 49 days (D49). The Intense Pulsed Light (IPL) treatment was performed with Thermaeye Plus (DEKA M.E.L.A. Spa, and worldwide distributed by MDS Medical Technologies SL, Spain), within the wavelength band range from 600 to 1200nm. The intensity of the treatment was 8J/cm2, on a contact surface of cool Sapphire, 48mm×13mm (6.2cm2), representing 49.6 Joules in each flash. According to the degree of skin pigmentation, it was adjusted the parameters of the number of micropulses, size, and time interval between them in each flash, which allows treating all patients on the Fitzpatrick scale, from I to VI.
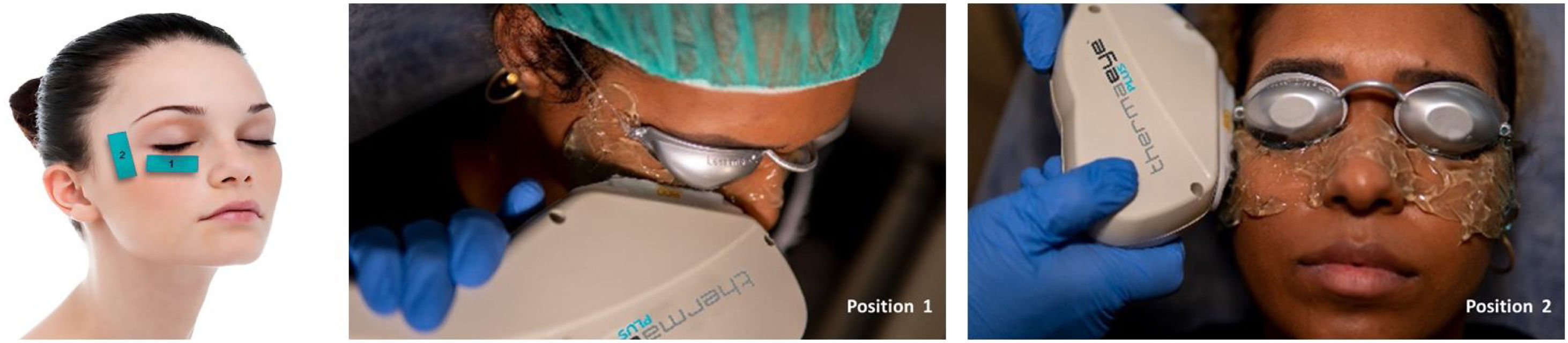
Before starting each treatment, the handpiece was calibrated and the energy and parameters selected must be entered so that if they are correct, the treatment can be started but, if a combination is entered that is not safe, the Thermaeye Plus software warns and will not let the handpiece shoot. The procedure begins with a preparation protecting the eye with opaque goggles and applying ultrasound gel on the patient's periocular area and lids (canthal to canthal) to spread the energy and to conduct the light providing a high degree of protection and efficacy. The IPL treatment is carried out with 6 flashes in each eye as shown in Fig. 1; 3 flashes on the skin below the lower eyelid (position 1) and 3 flashes on the canthal area (position 2), taking care to avoid overlap the same area alternating each flash between positions 1 and 2. Both eyes are treated in all the 4 sessions. At the end of each session, the protective goggles and gel are removed. Sun protection cream is recommended for the first 48h. The mechanical expression of the Meibomian glands after IPL treatment was not realized. It was allowed to maintain artificial tears but no more than 3 times a day.
Clinical assessmentSubjects were evaluated at six visits: on the same day just before the first IPL session, Baseline (D1), 14 days just before the second session (D14), 28 days, just before the third session (D28), 49 days, just before the fourth and last IPL session (D49), and 1 month (D1M) and four months (D4M) after the final IPL session. A general ophthalmic evaluation was performed at every visit for safety purposes, including best-corrected visual acuity (distance and near LogMAR), intraocular pressure (IOP), slit-lamp biomicroscopy of the anterior segment and funduscopy. The skin of the eyelids and the periocular area where the IPL handpiece was applied was also reviewed. Special care was taken to identify possible burns, redness, inflammation, and changes in pigmentation or eyelashes loss.
The clinical assessments included: symptoms evaluation, tear osmolarity (TO), non-invasive tear meniscus height (NITMH), non-invasive tear break-up time (NIBUT), corneal fluorescein staining (CFS), eyelid margin and Meibomian gland assessments, following the TFOS DEWS II recommendation. The Schirmer test was used only for the inclusion criteria in diagnosing patients with DED due to MGD (S1 T, without anesthesia during 5min and <5mm as cutoff). Symptoms were assessed in two different ways. (1) OSDI questionnaire: 12 items to assess ocular discomfort, effects of visual function and the impact of environmental triggers, on a scale from 0 (none) to 100 (maximum) and with a cutoff <13. (2) Symptoms Score: evaluate the severity of 10 ocular symptoms: dryness, foreign body sensation, watering, itchiness, visual fatigue, blurred vision, burning, light sensitivity, secretion disturbance and pain. For each symptom assessment, subjective scores were applied from 0=no discomfort to 10, the worst score. A total subjective symptom score was defined as the summation of these scores; thus, the minimum score was 0, and the maximum was 100, and <35 was the cutoff selected for normal values. The tests were used for every subject at every follow-up visit, from D1 (Baseline) to D4M (four months from the last IPL session).
Tear osmolarity (TO) was evaluated bilaterally from the inferior lateral tear meniscus with the Tear Lab system (Tar Lab Corp, San Diego, CA). Subjects were instructed not to instill drops for at least 2h before the examination. Adherence to this instruction was confirmed before taking measurements. The same diagnostic device was used for all subjects. A cutoff value of ≥310mOsm/L and an inter-eye difference of ≥8mOsm/L was used as a characteristic of DED. Non-invasive Tear Meniscus Height (NITMH) was evaluated with the Keratograph 5M (Oculus, Wetzlar, Germany), taking Infrared images of the tear meniscus. Three zones of the tear river were measured in each patient in the lower eyelid: temporal, central, and nasal eyelids, taking the average of the three measurements. The cutoff value was considered <0.20mm. Non-invasive BUT (NIBUT) was evaluated with the Keratograph 5M. The patient was asked to blink 3 times gently and then keep looking at the fixation point. Placido rings are reflected on the corneal surface, and the software analyzes different segments, and distortion in the reflected mires is recorded as a break in the tear film. The time when the first break in the tear film occurred is displayed. To obtain greater reliability, three measurements are performed, taking the average in each eye. The cutoff for normal values was >10s. and <5s for dry eye diagnosis. Corneal Fluorescein Staining (CFS) was examined with the slit-lamp under blue light illumination and a yellow filter. 2% Sodium fluorescein was instilled onto the bulbar conjunctiva, and the patient was asked to blink naturally without squeezing three to five times. Then the patient was asked to stare straight ahead without blinking. CFS was estimated using the Baylor grading scheme, staining of each of five zones of the cornea (central, temporal, nasal, superior, and inferior) was scored using the 5-point scale: 0 dots=0, 1–5 dots=1, 6–15 dots=2, 16–30 dots=3, and >30 dots=4. One point was added if there was a single area of confluent staining, and two points were added if there were at least two areas of confluent staining. The cutoff >10 was used to categorize DED as moderate to severe.
Eyelid Margin and Meibomian Glands were examined with the slit-lamp biomicroscopy and with the Keratograph 5M. Using to evaluate the eyelid margin the International Workshop on meibomian Gland Dysfunction criteria20: rounding of the posterior eyelid margin, irregularity of the eyelid margin, telangiectasia/vascularity of lid margin and blepharitis, Each sign scored from 0=normal or absent to 3=severe. Meibomian gland assessment was the sum of subscores, including (1) the number of plugged meibomian gland orifices within the central 1cm of the upper and lower eyelids (0=none to 3=80% occluded), (2) expressed secretion quality (from 0=clear to 3=pasty/solid), (3) the expressibility of the meibomian gland (0=minimal pressure to 3=heavy pressure) and (4) gland dropout. The Arita scoring system21 (meiboscore) was used to evaluate gland dropout in which each eyelid is examined for meibomian gland loss and scored from grade 0 (no loss of meibomian glands) through grade 3 (loss of more than two-thirds of the total meibomian gland area).
Safety evaluation was realized at every visit evaluating local and systemic adverse events. The skin areas around the eye were also assessed for the examination of depigmentation, blistering, swelling, redness, and hair loss at eyebrows, eyelashes, and forehead. A complete ophthalmology examination was realized at every visit.
Statistical analysisAll data were analyzed with the IBM SPSS Statistics for Windows (Version 25.0. Armonk, NY: IBM Corp). Descriptive statistics data were obtained for all patients and expressed as mean±standard deviation (SD). Continuous variables were tested for normality with the Shapiro–Wilk for small samples and with the Kolmogorov–Smirnov for large samples. Comparison of data before treatment and at each of the individual follow-ups was performed using paired analysis. Normally distributed continuous data were analyzed with the repeated measure analysis of variance (ANOVA) and the Bonferroni adjustment post hoc, that enabled comparison of data across the various time points, and paired analyses allowed comparison of pre- and posttreatment data at individual time points. For the associated qualitative variables, the Chi square independence test was performed. For statistical analysis, each eye has been evaluated as an independent sample, except in the cases of the OSDI questionnaire and Tear Osmolarity test, in which patients were evaluated. Results were considered statistically significant for p<0.05.
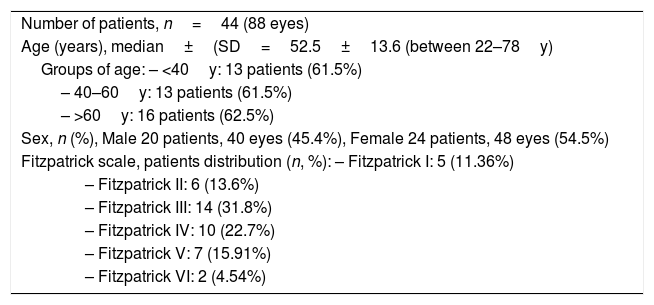
ResultsThe study included 44 consecutive patients (88 eyes), 20 males (40 eyes) and 24 females (48 eyes), with a mean age of 52.5±13.6 years, ranging from 22 to 78 years (Table 1). The distribution of patients according to the degree of skin pigmentation (Fitzpatrick classification) shows the majority correspond to intermediate grades, II, III, and IV, 30 patients (68.2%). There were also patients with grades I: 5 patients (11.36%), grade V: 7 patients (15.91%), and grade VI: 2 patients (4.54%).
Demographic data of patients with dry eye disease (DED) due to meibomian gland dysfunction (MGD) and the Fitzpatrick scale distribution.
| Number of patients, n=44 (88 eyes) |
| Age (years), median±(SD=52.5±13.6 (between 22–78y) |
| Groups of age: – <40y: 13 patients (61.5%) |
| – 40–60y: 13 patients (61.5%) |
| – >60y: 16 patients (62.5%) |
| Sex, n (%), Male 20 patients, 40 eyes (45.4%), Female 24 patients, 48 eyes (54.5%) |
| Fitzpatrick scale, patients distribution (n, %): – Fitzpatrick I: 5 (11.36%) |
| – Fitzpatrick II: 6 (13.6%) |
| – Fitzpatrick III: 14 (31.8%) |
| – Fitzpatrick IV: 10 (22.7%) |
| – Fitzpatrick V: 7 (15.91%) |
| – Fitzpatrick VI: 2 (4.54%) |
Before the study, all patients received previous treatment for dry eye, including artificial tears, warm compresses, and eyelid hygiene. In some cases, anti-inflammatory eye drops had been used at some point in their disease (68.2%). Still, following the exclusion criteria, no patient had used anti-inflammatory, antiallergy, or any other type of drops for a minimum of 1 month before to start the study. Of the 44 patients who started the study, 2 patients withdrew from the study for not attending all follow-up visits. The remaining 42 patients (84 eyes) completed the measurements and all the study requirements for analysis.
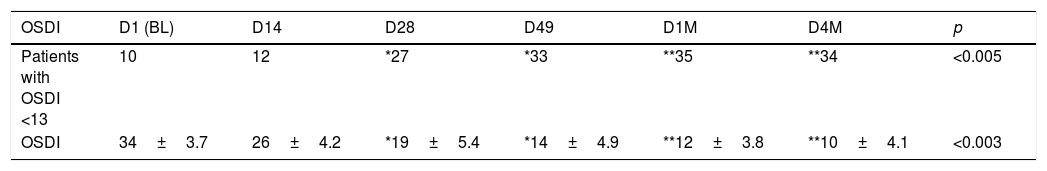
Clinical Symptoms in the OSDI questionnaire showed a significant decrease in total symptoms when compared to baseline D1 vs. the last visit D4M (p<0.003). The percentage of patients with a normal index <13 improved from 23.8% (10 patients) in D1 to 80.9% (34 patients) at D4M. The results in each of the follow-up visits are shown in Table 2, number of patients with OSDI <13 (normal) and absolute values of OSDI in all patients in each of the visits. The number of normal patients increases throughout the study, exceeding 50% (27 patients) after 28 days of starting treatment (D28), with a high degree of significance when performing the linear analysis of each one of the follow-up visits, p<0.005 (ANOVA test). The absolute values of the OSDI change from 34±3.7 in BL (D1) to 10±4.1, in the last visit D4M (D1 vs. D4M p<0.001, with Bonferroni correction), and p<0.003 in the linear evaluation of each one of the follow-up visits (ANOVA test).
Results of the OSDI questionnaire during the study, from the initial values in D1 (Baseline) to the last visits after 23 weeks, D4M.
| OSDI | D1 (BL) | D14 | D28 | D49 | D1M | D4M | p |
|---|---|---|---|---|---|---|---|
| Patients with OSDI <13 | 10 | 12 | *27 | *33 | **35 | **34 | <0.005 |
| OSDI | 34±3.7 | 26±4.2 | *19±5.4 | *14±4.9 | **12±3.8 | **10±4.1 | <0.003 |
The increase in patients with values within normality (<13) is appreciated, changing from 10 in D1 to 34 patients in D4M, with a high level of significance, p<0.005 (ANOVA, linear evaluation considering all follow-up visits). The OSDI values are also shown in each of the control visits. A progressive reduction was observed, reaching an average value of less than 13 in D1M, after 1 month from the last IPL session, maintained until the last visit of the study, D4M, with a significance value of p<0.005 when all the visits are evaluated (ANOVA test). The degree of significance when comparing the initial value before treatment (D1), with each of the control visits, was calculated with Bonferroni correction and represented as, * adjusted p<0.01 and ** adjusted p<0.001.
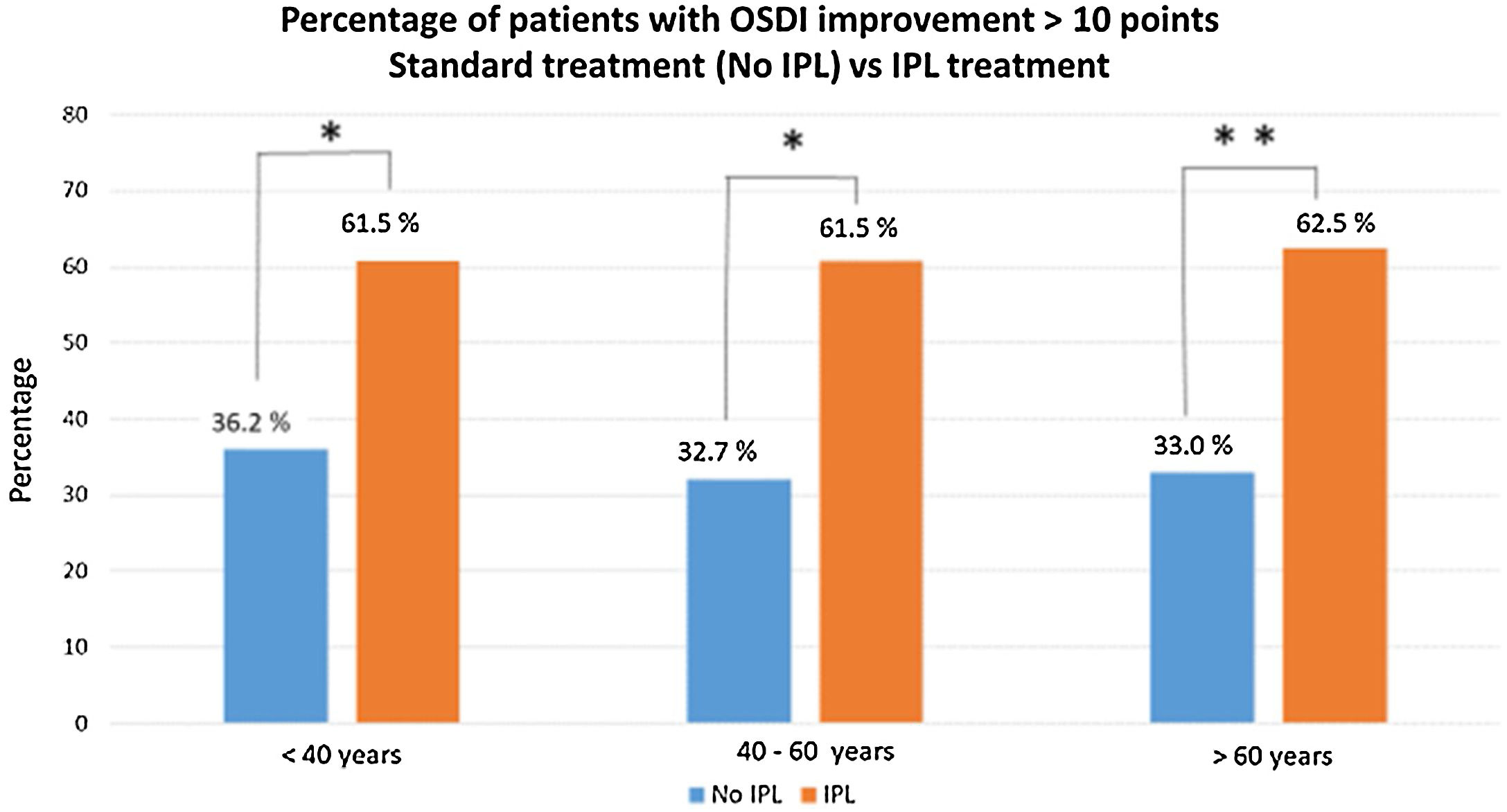
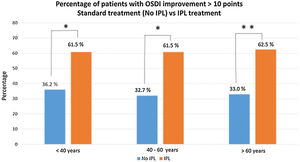
Comparing the percentage of patients with an improvement of more than 10 points in the OSDI questionnaire, between baseline D1 and the last visit D4M, the results evidence a high level of significance, p<0.005 (Chi square test). Fig. 2 shows these results, with the percentage of patients who have improved more than 10 points, divided into groups of age, <40 years (n=13), 61.5% (8 patients), between 40–60 years (n=13), 61.5% (8 patients) and, >60 years (n=16), 10 patients. There were no statistical differences between groups of age or gender.
Percentage of patients who have improved more than 10 points (D1 baseline vs. D4M last visit), divided into groups of age, and comparing results obtained in this study vs. previous data with similar conditions (demographics and DED-MGD), but without IPL treatment, only artificial tears and warm compresses (unpublished data). The comparative analysis (Chi square test), show significant differences between the two groups of treatment, No IPL vs. IPL treatment, with p<0.01 (*) in the group of age <40 years and p<0.001 (**) in the other 2 groups of age, between 40–60 years and >60 years.
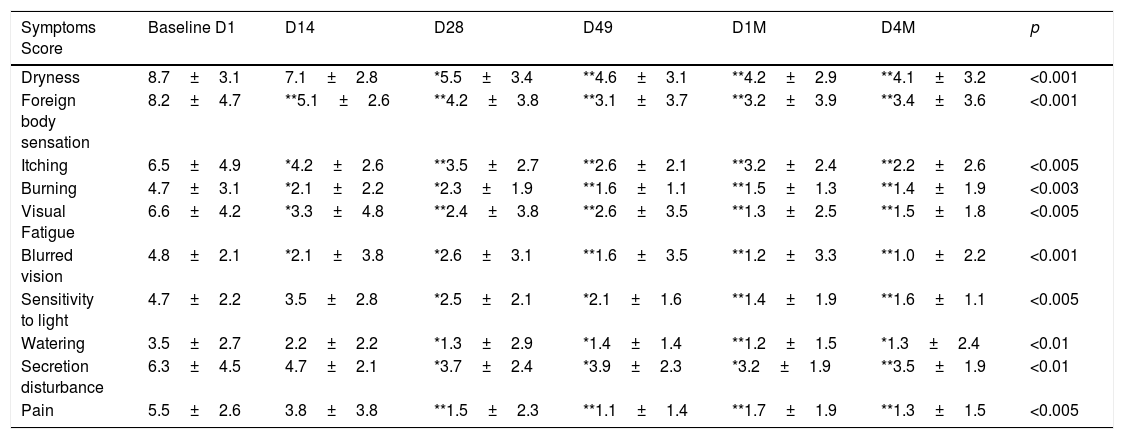
The results of the Symptoms Score are listed in Table 3. Compared to baseline (D1), all symptoms showed amelioration: D14, D28, D49, D1M, and D4M, 4 months after the last IPL session (p<0.005, ANOVA test). The most significant changes were observed in dryness, foreign body sensation (p<0.001), and pain (p<0.005). The other seven symptoms showed high significant p-value but slightly lower than the previous ones, as listed in Table 3. The longitudinal study over time shows that the improvement of the majority of symptoms begins 14 to 28 days after starting the IPL treatment (D14 and D28), with a degree of significance expressed in Table 3 on the quantitative values of each symptom in a given follow-up visit (*p<0.01, **p<0.001). Between the visits of D49 and D1M and D4M (1, and 4 months after the last IPL session), no significant difference was observed. Although there was no improvement during this period, the results remained stable during four months after the last IPL session. The combined evaluation of the 10 symptoms at each visit, showed an improvement from baseline, 59.5 (normal cutoff value <35), to 23.6 at the end of the treatment, D49 (p<0.003). The results keep stable until the end of the study, after 4 months from the last IPL session D4M, 20.3 (p<0.003).
Results of the symptoms score at each follow-up visit.
| Symptoms Score | Baseline D1 | D14 | D28 | D49 | D1M | D4M | p |
|---|---|---|---|---|---|---|---|
| Dryness | 8.7±3.1 | 7.1±2.8 | *5.5±3.4 | **4.6±3.1 | **4.2±2.9 | **4.1±3.2 | <0.001 |
| Foreign body sensation | 8.2±4.7 | **5.1±2.6 | **4.2±3.8 | **3.1±3.7 | **3.2±3.9 | **3.4±3.6 | <0.001 |
| Itching | 6.5±4.9 | *4.2±2.6 | **3.5±2.7 | **2.6±2.1 | **3.2±2.4 | **2.2±2.6 | <0.005 |
| Burning | 4.7±3.1 | *2.1±2.2 | *2.3±1.9 | **1.6±1.1 | **1.5±1.3 | **1.4±1.9 | <0.003 |
| Visual Fatigue | 6.6±4.2 | *3.3±4.8 | **2.4±3.8 | **2.6±3.5 | **1.3±2.5 | **1.5±1.8 | <0.005 |
| Blurred vision | 4.8±2.1 | *2.1±3.8 | *2.6±3.1 | **1.6±3.5 | **1.2±3.3 | **1.0±2.2 | <0.001 |
| Sensitivity to light | 4.7±2.2 | 3.5±2.8 | *2.5±2.1 | *2.1±1.6 | **1.4±1.9 | **1.6±1.1 | <0.005 |
| Watering | 3.5±2.7 | 2.2±2.2 | *1.3±2.9 | *1.4±1.4 | **1.2±1.5 | *1.3±2.4 | <0.01 |
| Secretion disturbance | 6.3±4.5 | 4.7±2.1 | *3.7±2.4 | *3.9±2.3 | *3.2±1.9 | **3.5±1.9 | <0.01 |
| Pain | 5.5±2.6 | 3.8±3.8 | **1.5±2.3 | **1.1±1.4 | **1.7±1.9 | **1.3±1.5 | <0.005 |
The linear analysis evaluating visits from D1 (baseline), before first IPL sessions and D14, D28, D49 and 1 month, D1M and 4 months, D4M after the last IPL session (ANOVA test), show that the most significant changes were observed in dryness, and foreign body sensation, both with p<0.001, and pain: p<0.005. The longitudinal study over time (Bonferroni adjustment), shows that the improvement of the majority of symptoms begins at D14 and D28, with a degree of significance of, * adjusted p<0.01 and ** adjusted p<0.001, in every follow-up visits vs. D1 Baseline.
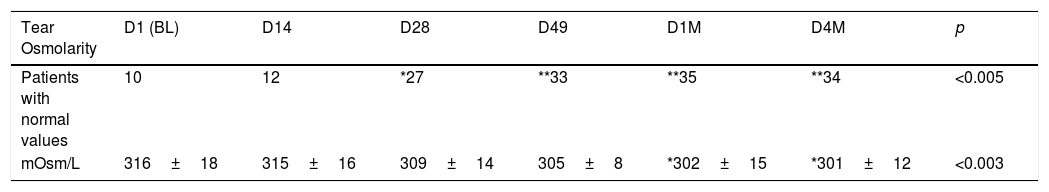
Tear Osmolarity (TO) showed significant improvement comparing the baseline values before starting the treatment, 316±18mOsm/L vs. the final visit (D4M), 301±12mOsm/L, p<0.007. Considering normal values ≤310mOsm/L or an inter-eye difference of ≤8mOsm/L, the normal values at the beginning of the study was 23% (10 patients) and at the end 81% (34 patients), p<0.001. The results of the evolution over time in each of the follow-up visits are shown in Table 4. It is observed how the number of patients with normal values increases, exceeding 50% of patients (26) after 3 weeks of starting treatment, with a high level of significance p<0.005 in the linear analysis. Additionally, it is observed that the osmolarity values are improving throughout the study, reaching values below 310mOsm/L at visit D28 (309±14mOsm/L). Linear statistical analysis, evaluating all visits also shows a high level of significance, p<0.003 (ANOVA test). There were no significant differences when comparing males and females and between the three groups of age, <40y, 40–60y and >60y at each of the follow-up visits.
Tear osmolarity results are shown throughout the study, in each of the visits, the osmolarity values (mOsm/L), and the number of patients with normal values (≤310mOsm/L or with differences between the two eyes ≤8mOsm/L).
| Tear Osmolarity | D1 (BL) | D14 | D28 | D49 | D1M | D4M | p |
|---|---|---|---|---|---|---|---|
| Patients with normal values | 10 | 12 | *27 | **33 | **35 | **34 | <0.005 |
| mOsm/L | 316±18 | 315±16 | 309±14 | 305±8 | *302±15 | *301±12 | <0.003 |
In both cases, an improvement in the results is observed, with p<0.005 concerning the osmolarity values and p<0.005 to the number of patients with values within normality, increasing from 10 patients before starting IPL treatment (D1) to 34 patients of the 42 who completed the study, at the last visit D4M (23 weeks after the start). Statistical analysis was performed with the ANOVA test and the Bonferroni correction to compare the initial value, D1, with each of the values in the follow-up visits, representing the degree of significance as * adjusted p<0.01 and ** adjusted p<0.001.
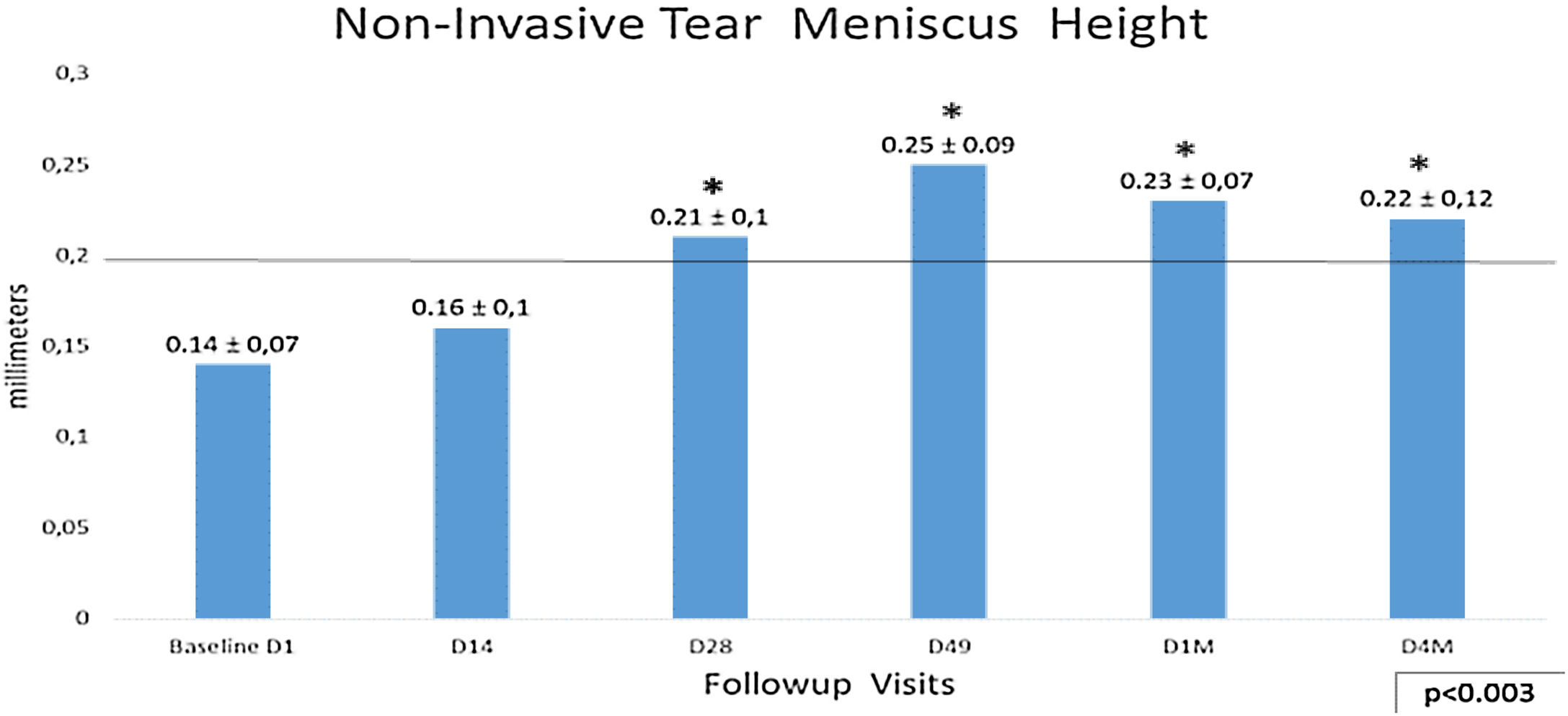
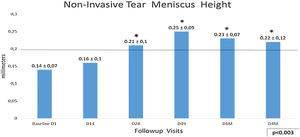
Non-invasive Tear Meniscus Height (NITMH) showed a linear improvement from baseline 0.14±0.07mm to D49 0.25±0.09mm, p<0.003. The trend from D49 to last visit D4M (0.22±0.12mm), was toward the stabilization with values no statistically significant (Fig. 3), showing a tendency similar to other tests in this study. Regarding percentages of eyes with values below cutoff, at the baseline visit, there were 80.9% (68 eyes) and only 14.3% (12 eyes) at D49 (last IPL sessions), and 7.14% (6 eyes) at the last visit, D4M.
Non-invasive Tear Meniscus Height values with 0.20mm as cutoff. The longitudinal analysis shows a linear improvement from baseline to D49, last IPL session (p<0.003,) with a tendency to stabilize these results to the final visit after 4 months, D49 vs. D4M, p no significant (Bonferroni correction). Statistical analysis was performed with the ANOVA test and the Bonferroni correction to compare the initial value with each of the values in the follow-up visits, representing the degree of significance as * adjusted p<0.01 and ** adjusted p<0.001.
Analyzing the NITMH respect to the groups of age by means the Bonferroni correction, in the group of eyes corresponding to patients under 40 years, there was an improvement from the baseline D1, 0.19±0.10mm to the last visit D4M, 0.22±0.12mm, but it did not show significant differences. The eyes corresponding to the group of patients over 60 years, showed the greatest difference, from 0.14±0.05mm before the treatment D1, to 0.27±0.07mm, at the final control D4M, p<0.0007. No differences were obtained comparing males and females.
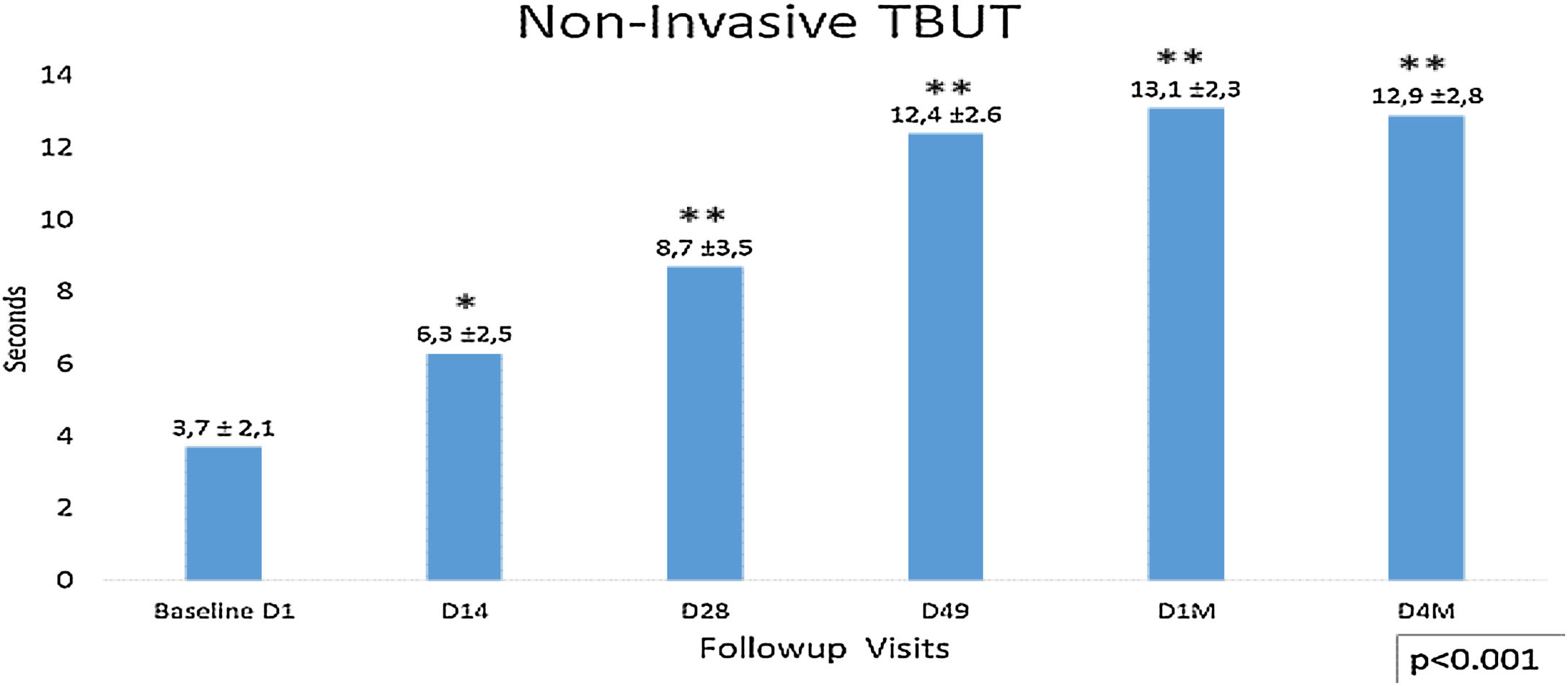
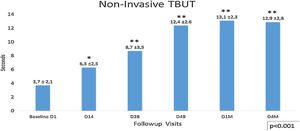
Non-invasive tear break time (NITBUT) increased during the follow-up visits after the first IPL season D1 (3.7± 2.1) to the last control D4M (12.9±2.8) p<0.001. At days 14 (6.3±2.5) and 28 (8.7±3.5), significant improvement of the NIBUT is observed regarding the baseline (3.7±2.1), p<0.006. The most important difference was between D28 and D49, p<0.001 (Fig. 4). This tendency stabilizes between visits D49 (12.4±2.6) and last visit D4M (12.9±2.8), and although a slight improvement is observed, there were no significant differences during this time (D49 vs. D4M). The analysis of the percentage of eyes concerning the 5s cutoff for dry eye, showed that 57.3% of eyes present higher values, >5s (no dry eye), at the beginning of the study (D1 Baseline), increasing to 100% in D49 and maintaining this percentage until the last visit, 4 months after finishing the IPL treatment. No differences between males and females were observed.
Non-invasive Tear Break Time (NITBUT), with <5s as dry eye cutoff. It shows lineal increased values after the first IPL season D1 (3.7±2.1s) to the last control D4M (12.4±2.6s), p<0.001 (ANOVA test). At days 14 (6.3±2.5s) and 28 (8.7±3.5s), significant improvement of the NIBUT is observed regarding the baseline (3.7±2.1s), p<0.006. The most important difference was between D28 and D49, p<0.001. This tendency change between visits D49 (12.4±2.6s) and last visit D4M (12.9±2.8s), and although a slight improvement is observed, there were no significant differences during this time (D49 vs. D4M). Statistical analysis was performed with the ANOVA test and the Bonferroni correction to compare the initial value with each of the values in the follow-up visits and between different visits, representing the degree of significance as * adjusted p<0.01 and ** adjusted p<0.001.
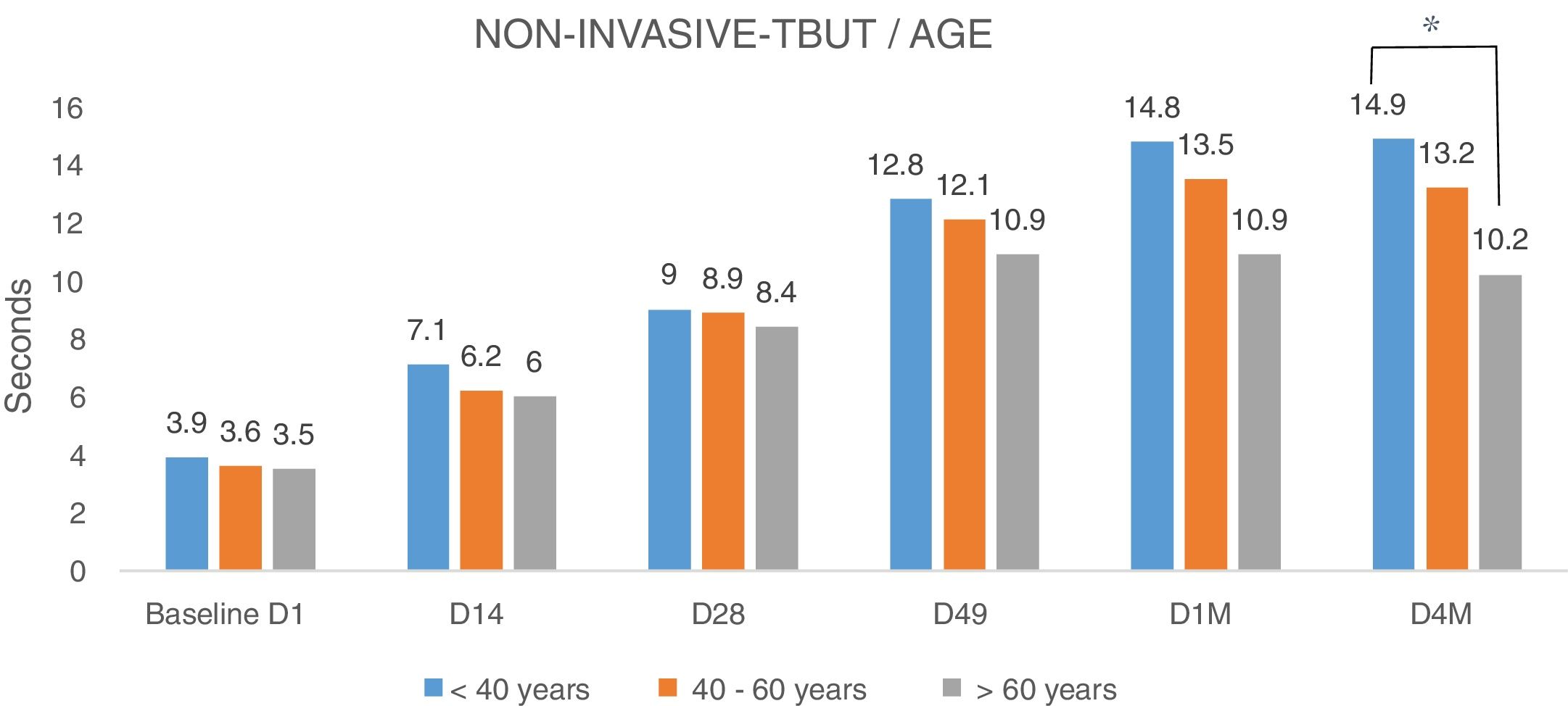
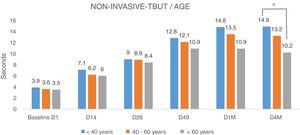
Analyzing the NITBUT according to the age of patients (Fig. 5), divided into three groups (<40 years, between 40 and 60 years and >60 years), there were no differences (Fig. 5). The NITBUT values of the eyes in the three groups are very similar at the baseline D1, before treatment, without statistical significance. The comparison between baseline visit and the last visit D4M, 4 months after the last IPL session, in group <40 years, the difference was highly significant, p<0.001, the group between 40 and 60 years, the degree of significance was lower, p<0.005 and in the group of more than 60 years, the difference was minimally significant, p<0.01. Differences between the three groups of age in the last control, D4M, showed a higher degree of significance when comparing the groups of younger age, <40 years, and the group over 60 years, p<0.005 (Bonferroni correction).
NITBUT results of eyes evaluated and divided into three groups of age, <40y, between 40 and 60y and >60y. Values in the three groups are very similar at the baseline D1, before treatment, without statistical significance. The differences increased progressively toward the last visit D4M, 4 months after the last IPL session. In group <40y, the difference was highly significant, p<0.001; the group between 40 and 60y, the degree of significance was somewhat lower, p<0.005; and in the group of more than 60y, the difference was minimally significant, p<0.01. At the last control, D4M, the differences between groups of younger age, <40y, and group >60y, show a higher degree of significance, p<0.005 (*). Statistical analysis was performed with the ANOVA test and the Bonferroni correction to compare the initial value with each of the values in the follow-up visits and between different visits.
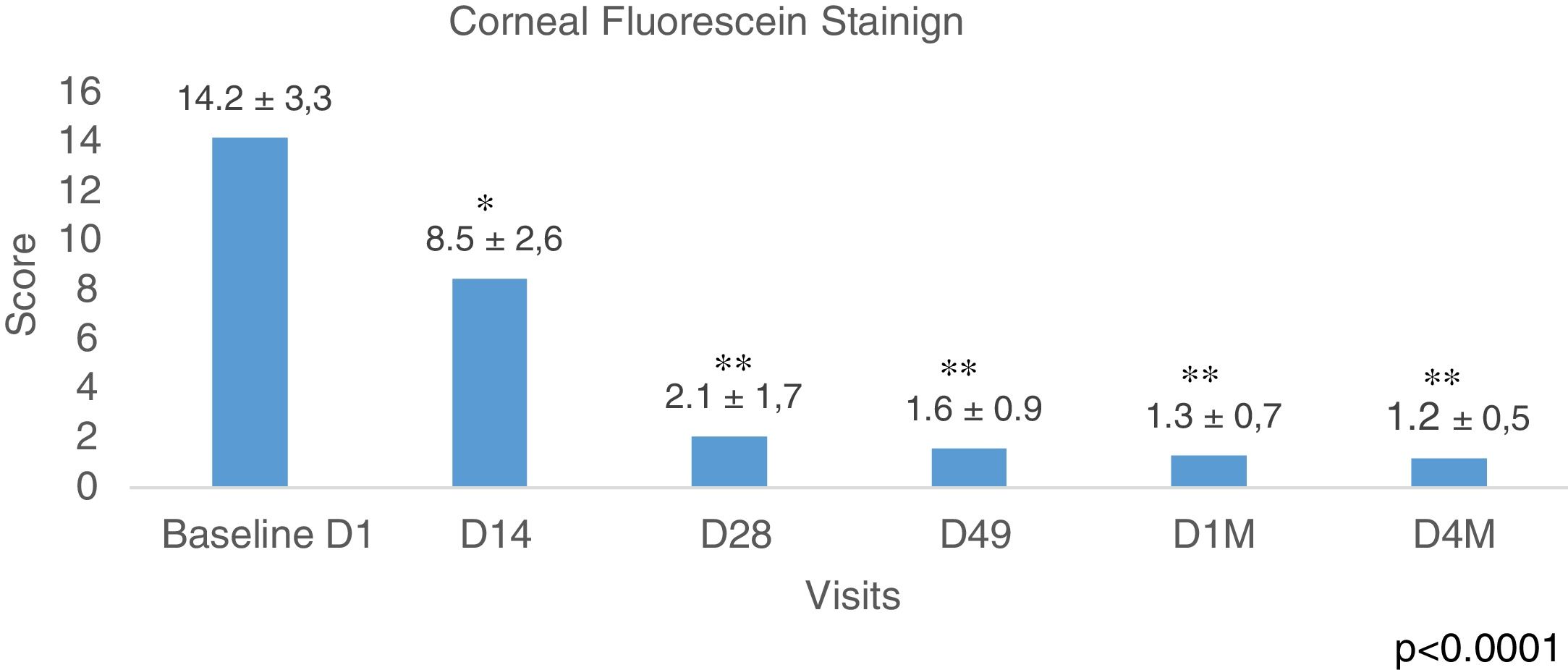
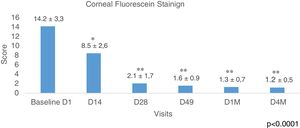
Corneal Fluorescein Staining was evaluated using the Baylor grading scheme and <10 as a normal cutoff value. Fig. 6 shows the results of all the eyes evaluated showing a longitudinal reduction of values during the study, p<0.0001 (ANOVA test). The improvement was from the beginning 14.2±3.3 to 8.5±2.6 after 2 weeks (D14) of the first IPL session, p<0.01 (Bonferroni correction). This data is important because it means that since the second week, it was already obtained results below the normal value (<10). The most relevant improvement was obtained at D28, 2.1±1.7, (p<0.001), and these values remained similar during the study to the last control D4M, 1.2±0.5, 4 months after finishing the IPL treatment. The analysis of the percentages of eyes with values belong the cutoff <10 (normal values), showed at the baseline visit 40 eyes, which represents 47.6% and this value increased to 78 eyes, 92.8% at visit D49, before the last session of IPL, improving up to 100% of the eyes in the last visit, 4 months after the end of the IPL sessions (84 eyes corresponding to the 42 patients of the study). No differences were obtained when comparing males and females.
Fluorescein staining. CFS (Baylor grading scheme and >10 as normal cutoff value). Data show a longitudinal reduction of values during the study, from D1 (before IPL treatment) to D4M (last visit, 4 months after last IPL session), p<0.0001 (ANOVA test). The improvement was registered after 2 weeks from the first IPL session, p<0.01. The most relevant improvement was obtained at D28, p<0.0001, and these values remained similar during the study to the last control D4M, 4 months after finishing the IPL treatment. Time course of CFS before, during, and after the IPL treatment, * adjusted p<0.01, ** adjusted p<0.001 versus corresponding pretreatment Baseline D1 (Bonferroni correction).
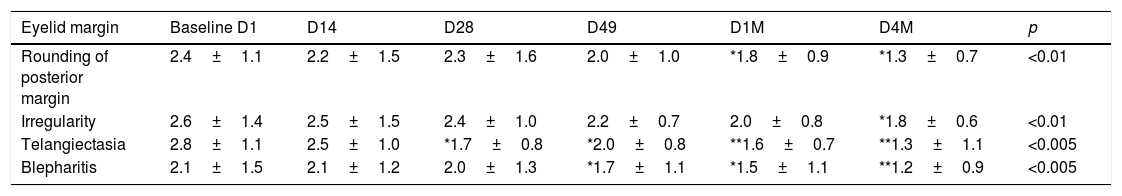
Eyelid Margin and Meibomian Gland Assessments were evaluated at every visit in each eye: rounding of the posterior eyelid margin, irregularity of eyelid margin, telangiectasia/vascularity of eyelid margin, and blepharitis. Each sing scored from 0=normal or absent to 3=severe. The results are listed in Table 5. Compared to baseline, all these signs were significantly improved after the treatment. The most significant improvement was for telangiectasia/vascularity and blepharitis with p<0.005 in both cases. For the other two signs, rounding, and irregularity eyelid margin, the significance was p<0.01. The most significant difference between visits during the study was between D49, after 4 sessions of IPL and D1M, 1 month after the last IPL session (p<0.008), while between D1M and D4M, no significant difference was observed.
Eyelid margin assessments.
| Eyelid margin | Baseline D1 | D14 | D28 | D49 | D1M | D4M | p |
|---|---|---|---|---|---|---|---|
| Rounding of posterior margin | 2.4±1.1 | 2.2±1.5 | 2.3±1.6 | 2.0±1.0 | *1.8±0.9 | *1.3±0.7 | <0.01 |
| Irregularity | 2.6±1.4 | 2.5±1.5 | 2.4±1.0 | 2.2±0.7 | 2.0±0.8 | *1.8±0.6 | <0.01 |
| Telangiectasia | 2.8±1.1 | 2.5±1.0 | *1.7±0.8 | *2.0±0.8 | **1.6±0.7 | **1.3±1.1 | <0.005 |
| Blepharitis | 2.1±1.5 | 2.1±1.2 | 2.0±1.3 | *1.7±1.1 | *1.5±1.1 | **1.2±0.9 | <0.005 |
The four signs evaluated showed a significant improvement after the IPL treatments. The most significant improvement was for telangiectasia/vascularity and blepharitis p<0.005 in both cases (ANOVA test). The most significant difference between visits during the study was between D49, after 4 sessions of IPL and D1M, 1 month after the last IPL session, p<0.008 (Bonferroni correction). In contrast, between D1M and D4M, no significant difference was observed. Statistical analysis was performed with the ANOVA test and the Bonferroni correction to compare the initial value with each of the values in the follow-up visits, * adjusted p<0.01, ** adjusted p<0.001.
Telangiectasia and blepharitis are the two sings with the best score. The percentage of cases without telangiectasia, improved from 23.8% (20 eyes) at D1 to 45.2% (38 eyes) at D4M, p<0.002. The percentage of eyes with no blepharitis improved from 8 eyes, 9.5% at D1 to 36 eyes, 42.8% at D4M, p<0.0005.
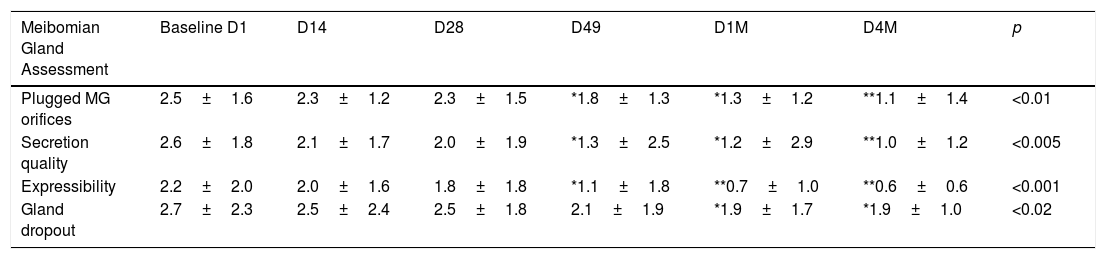
Meibomian gland assessment in each eye includes (1) number of plugged meibomian gland orifices (PMGO) within the central area, 1cm of the upper and lower eyelids, (2) quality of expressed secretion, (3) the expressibility of the meibomian gland and (4) gland dropout. Results correspond a score between 0 to worst 3, and are listed in Table 6. Continuous improvement from Baseline to the last visit at 4 months (D4M) was registered. The number of plugged meibomian gland orifices decreased during the study (p<0.01, ANOVA test). Comparing the baseline D1 (2.5±1.6) and the final visit D4M (1.1±1.4), a p<0.01 was obtained (Bonferroni correction). The comparison of the quality of meibomian gland secretion at D1 (2.6±1.8) vs. D4M (1.0±1.2), showed a general improvement, p<0.005. The degree of expressibility changed from D1 (2.2±2.0) to D4M (0.6±0.6), p<0.001. Evaluating these two signs, quality and expressibility, the most significant changes were between visits D28 just before the third IPL session and D49, before the fourth and last IPL session, p<0.005 (Table 6). Between D49 and the last visit D4M, the results were stable with no significant difference for both signs, quality, and expressibility.
Meibomian gland assessment results show a continuous improvement from D1 (before IPL treatment) to the last visit D4M, 4 months after the last IPL session.
| Meibomian Gland Assessment | Baseline D1 | D14 | D28 | D49 | D1M | D4M | p |
|---|---|---|---|---|---|---|---|
| Plugged MG orifices | 2.5±1.6 | 2.3±1.2 | 2.3±1.5 | *1.8±1.3 | *1.3±1.2 | **1.1±1.4 | <0.01 |
| Secretion quality | 2.6±1.8 | 2.1±1.7 | 2.0±1.9 | *1.3±2.5 | *1.2±2.9 | **1.0±1.2 | <0.005 |
| Expressibility | 2.2±2.0 | 2.0±1.6 | 1.8±1.8 | *1.1±1.8 | **0.7±1.0 | **0.6±0.6 | <0.001 |
| Gland dropout | 2.7±2.3 | 2.5±2.4 | 2.5±1.8 | 2.1±1.9 | *1.9±1.7 | *1.9±1.0 | <0.02 |
The quality of meibomian gland secretion and the degree of expressibility (p<0.001), show better scores regarding the other two signs, plugged orifices and gland dropout (p<0.01 and p<0.02). The most significant change was between visits D21 just before the third IPL session, and these results were maintained stables to the end of the study 23 weeks from D1. Statistical analysis was performed with the ANOVA test and the Bonferroni correction to compare the initial value with each of the values in the follow-up visits, * adjusted p<0.01, ** adjusted p<0.001.
No side effects appeared with Thermaeye Plus demonstrating safety, even in patients with pigmentation type IV, V, and VI on the Fitzpatrick scale. There were no adverse events as depigmentation, blistering, swelling, redness, and hair loss at the eyebrows and eyelash during the evaluation.
DiscussionThis is the first study of IPL in Ophthalmology to include patients with all degrees of skin pigmentation (I to VI on the Fitzpatrick scale). The data shows that there were no complications in patients with dark skin, and the efficacy was similar to those patients with lighter skin. This is important in the moderate to high brown-dark skin population to perform safely and effectively IPL treatment in all patients. Other IPL devices reported complications in these patients, who are excluded in some studies.22–26 In the protocol, Thermaeye Plus was used in a spectrum from 600nm and with low energies (8J/cm2), adjusting the parameters of the number of micropulses, size and time interval between them in each flash according to the degree of skin pigmentation, which allows treating all patients on the Fitzpatrick scale, from I to VI. The combination of low energy micropulses and the possibility to adjust different parameters explain why micropulses has a lower absorption by melanin from the skin and allows light to pass and reach deeper layers arriving at the meibomian glands. These parameters allow the same efficacy but reducing the risk of skin complications.
The results obtained in this study show improvement of patients suffering from DED due to MGD similar to other trials with patients treated with IPL.12,13,21,27–31 80.9% of patients present improvement in the OSDI questionnaire and between 61.5–62.5%, amelioration of more than 10 points of the OSDI questionnaire, with no differences between the groups of age. Clinically, an improvement of over 90% was obtained in most of the tests carried out in this study. Similarly, Toyos12 presented an improvement of DED symptomatology in 93% of cases and clinical signs in 87%, although it is a retrospective study where basically patients with clear skin were treated (grades I, II and III, excluding patients with grades IV, V and VI in the Fitzpatrick scale), with energies that reached up to 20J/cm2, very different from the parameters used in our study, 8J/cm2 and in which patients with degrees of pigmentation from I to VI on the Fitzpatrick scale were included. Vegunta et al.29 showed that the combination of IPL and meibomian gland expression (MGX) improved dry eye symptoms in 89% of patients, and meibomian gland function in 77% of patients, similar results to those obtained in our study but with the exception that the meibomian gland expression maneuver was not performed.
All symptoms improved along the study, especially the foreign body sensation, dryness, and pain. These results could be related to the decrease of the inflammatory status since inflammation affects the perception of pain16 (neuropathic pain). In this line, Karaca et al.,30 referred amelioration of symptoms two hours after the IPL treatment and they speculated with a neurological effect of the IPL.
The IPL effect starts from the second week of treatment, improving throughout the study, markedly from the second session of IPL, after 28 days (D28). The trend has a lineal improvement until 1 month (D1M) to the fourth and last session of IPL treatment, after which a slight improvement is observed, continuing until the last visit, after 4 months (D4M) of the last IPL session. It is remarkably that symptoms and clinical signs were stable during the 4 months after finishing the IPL sessions. These facts could be interpreted as this treatment has an acceptable duration, and it is unnecessary to repeat frequently new IPL sessions, as has been suggested by other authors.27,29 There were no differences when comparing patients according to gender and age groups.
The improvement in vision was attributed to the amelioration of the quality of tear film, tear osmolarity, lipid layer, BUT, and the corneal status (corneal staining), similar to other studies.14,21,28 Respect to tear osmolarity, there were only 31% (13 patients) with normal values at the beginning of the study and increased to 88% (37 patients) at the end of the study. Regarding the tear meniscus height, percentages of eyes with abnormal values, below cutoff at the baseline visit, there was 80.9% (68 eyes) and only 14.3% (12 eyes) at D49 (last IPL sessions), and 7.14% (6 eyes) at the last visit, D4M. In the same line, NITBUT show 57.3% of eyes present higher values, >5s (no dry eye), at the beginning of the study (D1 Baseline), increasing to 100% in D49 and maintaining this percentage until the last visit, 4 months after finishing the IPL treatment. Finally, the corneal staining test (CSF), show the percentages of eyes with values belong the cutoff <10 (normal values), at the baseline visit, 40 eyes, which represents 47.6% and this value increased to 78 eyes, 92.8% at visit D49, before the last session of IPL, improving up to 100% of the eyes in the last visit, 4 months after the end of the IPL sessions (84 eyes corresponding to the 42 patients of the study).
Patients with DED have high levels of inflammatory molecules in the tears and ocular surface.9,32 IPL has the potential to interfere with this inflammatory cycle, by upregulation of anti-inflammatory cytokines, or downregulation of proinflammatory cytokines, by downregulation of tumor necrosis factor-α (TNF-α).16,33 Another argument is the thrombotic effect of IPL on small blood vessels28 around the meibomian glands and telangectasic vessels in lids and eyelid margins. It supposes a reduction in the reservoir of inflammatory mediators and, consequently, eliminating a major source of inflammation from the eyelids and meibomian glands.34 IPL may reduce the meibum density inside the glands, changing from a state of viscous gel in MGD to fluid, facilitating its release. Therefore, the analysis of meibomian glands is relevant to evaluate the effect of IPL. Cheng and Xue13,31 referred good results after IPL treatment showing the improvement related to the microstructures of the meibomian glands with in vivo non-invasive confocal microscopy (INICM). In our study, the meibomian gland was analyzed with infrared images (Keratograph 5M) and although the results showed slight improvement, we believe that in vivo confocal microscopy provides more details to analyze these structures, this is one of the limitations of this study.
A significant amelioration was observed within the eyelid margin analysis, the number of plugged orifices, and the secretion quality and expressibility of the glands, similar to other studies.13,27–31 Vora and Gupta27 reported improvement of lid margin edema, in the meibum quality score, and found a significant increase in the oil flow score and tear film break-up time, and a decrease in ocular surface disease index scoring. In our study, telangiectasia and blepharitis are the two sings with the best score. The percentage of cases without telangiectasia, improve from 23.8% (20 eyes) at D1 to 45.2% (38 eyes) at D4M, p<0.002. The percentage of eyes with no blepharitis improve from 8 eyes, 9.5% at D1 to 36 eyes, 42.8% at D4M, p<0.0005.
There were no differences when comparing patients and eyes according to gender and age groups. The improvement in eyes after IPL treatment was very similar in patients <40 years, compared to patients over 60 years of age with the only difference of NITMH and NITBUT. NITMH was better in older patients, and NITBUT was better in younger patients. In NITMH the improvement in older patients (32 eyes) could be due to a higher increase in tear volume in patients who initially had lower values. In NITBUT, the improvement in younger patients (26 eyes) could be related to the worst condition of the meibomian glands and the inflammatory status in younger patients at the beginning and their improvement after the IPL treatment, determining secondary the quality of tears and the NITBUT. These results must be interpreted with some caution because the number of eyes in each group was small, a limitation of the study.
LimitationsThere are several limitations to this study. First, the number of enrolled patients is limited, and may not have been sufficiently large to determine differences between groups of age or sex. Second, this trial was a single-arm, lacking a non-treatment control group, the placebo effect, and the risk of investigator bias could have influenced the results. Third, we used fixed treatment energy (8J/cm2), which might have influenced the treatment outcomes. Fourth, we have not carried out a specific study for inflammation and neurotrophic factors. Fifth, it would be necessary to study the Meibomian glands with confocal microscopy to visualize more precisely changes than those provided by infrared meibography, including the effect of IPL on Demodex and corneal nerves. Further studies may lead to a better understanding of the mechanism of function of IPL technology.
ConclusionThermaeye Plus, a new IPL for ophthalmic use, is a safe and effective treatment in patients with DED secondary to MGD, showing improvement from the second week of treatment and maintaining until the last visit, 4 months after the last IPL session (23 weeks of follow-up). It improves dryness, foreign body sensation, and pain (80.9% of patients). Clinical sings, telangiectasia and blepharitis also improved, more than 90%. Patients with pigmented skin (Fitzpatrick V or VI) have been treated with no side effects. It is very relevant, especially to apply in brown-dark skin populations since complications with other IPL devices have been reported.
Conflicts of interestThe authors have no conflicts of interest to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.