Keratoconus (KC) is a corneal ectasia characterised by steepening corneal curvature, changes in refractive error and corneal thickness that result in visual impairment. Early signs of KC include displacement of the thinnest part of the cornea from the central position, changes in the corneal epithelial layer cell distribution, variations in the anterior corneal astigmatism/posterior corneal astigmatism relationship and a variation in corneal thickness. It is important that we review the corneal imaging methods for the diagnosis of preclinical KC.
MethodAn online literature search was carried out on PubMed. Only publications detailing corneal assessment procedures were considered for this review and any publication on instruments that did not generate KC predictability indices were also excluded from the review. The 308 publications were reviewed.
DiscussionCorneal assessment techniques, with the ability to characterise both the anterior and posterior corneal surfaces, are invaluable in the diagnosis of pre-clinical KC. Reflection based and elevation based corneal imaging systems should be used in conjunction with other assessments such as higher order aberration measuring systems to improve sensitivity and reliability in the diagnosis of pre-clinical KC. Ultra high resolution ultrasound can detect pre-clinical KC. The ability to asses both the epithelium and endothelium makes anterior surface optical coherence tomography a superior technique for pre-clinical KC diagnosis. There is a positive correlation between central corneal thickness and corneal hysteresis. Corneal biomechanics should be considered in conjunction with other corneal assessments in the diagnosis of pre-clinical KC.
El queratocono (KC) es una ectasia corneal caracterizada por incremento de la curvatura corneal, cambios del error refractivo y espesor corneal que deriva en trastornos visuales. Los primeros signos de KC incluyen desplazamiento de la posición del punto más delgado de la córnea desde su posición central, cambios en la distribución de las células epiteliales de la córnea, variaciones en términos de la relación entre astigmatismo corneal anterior y posterior, y variación del espesor corneal. Es importante revisar los métodos de análisis por imagen corneal para diagnosticar el KC pre-clínico.
MétodoSe realizó una búsqueda online en la literatura científica en PubMed. Para esta revisión consideramos únicamente las publicaciones que detallaban los procedimientos de valoración corneal, excluyendo de la revisión cualquier publicación sobre los instrumentos que no generaban índices de predictabilidad del KC. Se revisaron 308 publicaciones.
DiscusiónLas técnicas de valoración corneal con capacidad para caracterizar las superficies corneales anterior y posterior son inestimables para el diagnóstico del KC pre-clínico. Deberán utilizarse las técnicas de imagen corneal basados en reflexión y elevación, junto con otro tipo de valoraciones tales como los sistemas de medición de aberraciones de alto orden, para mejorar la sensibilidad y fiabilidad del diagnóstico del KC pre-clínico. La ecografía de ultra alta resolución puede servir también para detectar el KC pre-clínico. La capacidad de valorar tanto el epitelio como el endotelio hace de la tomografía de coherencia óptica una técnica superior para el diagnóstico del KC pre-clínico. Existe una correlación positiva entre el espesor corneal central y la histéresis corneal. Deberá considerarse la biomecánica corneal, junto con otros métodos de valoración corneal, para el diagnóstico del KC pre-clínico.
Keratoconus (KC) is a well-documented1–3 corneal ectasia characterised by steepening corneal curvature, changes in refractive error and changes in corneal thickness that result in visual impairment. Although the first appearance of KC in the literature dates back to the 18th century, Nottingham provided the first detailed understanding in 1854.2 The diagnosis of KC involves identification of the following signs during a clinical eye exam; scissors reflex on retinoscopy, irregular astigmatism, steep keratometry readings often accompanied by distorted mires, topography and tomography map changes, reduced central corneal thickness on pachymetry and slit lamp signs such as Vogt’s striae, Fleischer’s ring, Rizzuti’s sign, Munson sign and stromal scarring.1
Different stages of the condition have been described using various terms including pre-clinical KC, sub-clinical KC, form fruste KC and clinical KC. Pre-clinical and sub-clinical KC are used interchangeably to describe the earliest form of the condition in which topography changes associated with KC are absent and the vision can still be corrected to normal levels with conventional methods such as spectacles. As KC is an asymmetrical condition, one eye develops clinical KC before the other. Clinical KC is characterised by the aforementioned slit-lamp findings associated with KC and positive KC findings on topography. Form fruste KC is used to describe the fellow eye without clinical KC signs.
The prevalence of KC ranges from less than 1 per 100 000 to as high as 229 per 100 000.1,2 It is important to note that the study that demonstrated the lowest prevalence used keratometry measurements alone in diagnosing KC and those that yielded higher levels of prevalence combined keratometry with another diagnostic assessment such as retinoscopy or topography for the diagnosis of KC.1 The lower reported prevalence may have been due to under-diagnosis of the condition due to equipment limitations, therefore denoting the importance of a comprehensive assessment in the diagnosis of KC. Incidence ranges between 1.3 and 25 per 100 000 each year.1,2,4 The increased incidences more recently reported were established following a detailed clinical exam which included keratometry, refraction and slit lamp examinations.5,6 This provides additional evidence that an improvement in investigation techniques results in increased sensitivity in the diagnosis of the condition.
Current research has the condition presenting in the pubescent years1,7 It affects both men and women but is thought to develop earlier and progress more rapidly in men compared to women.8 However, women seem to report more adverse effects on their quality of life as noted on a National Eye Institute Visual Function Questionnaire 25 evaluation.8 In a Dutch population the prevalence was shown to be higher amongst the men.4 Paediatric KC has been documented to be more aggressive as it is typically characterised by high rates of progression.9
There is no cure for this progressive condition but it can be managed conservatively through the use of spectacles and/or contact lenses to aid vision in the early stages. It may also be managed surgically with procedures such as collagen crosslinking, the use of intacs and penetrating keratoplasty.10,11 Collagen crosslinking is a relatively new procedure with the longest follow up recorded to date being ten years post intervention.12 It has been shown to halt the progression of KC, decrease corneal steepness by up to 2D and even improve both uncorrected and best corrected visual acuity post treatment.10,13 These findings make collagen crosslinking a valuable treatment option in the long-term management of KC. Early diagnosis and intervention remains key for positive prognosis. A timely diagnosis means the implantation of intra-corneal ring segments (ICRS) into the corneal stroma can still be considered. ICRS have been shown to reduce corneal astigmatism, regularise topography maps and decrease the amount of corneal aberrations seen for KC patients thus improve visual performance and quality of life.14,15
Normal healthy cornea comprises of six layers,16 namely the Epithelium, Bowman’s layer, Stroma, Descemet’s membrane, Dua layer and Endothelium. Early signs of KC include displacement of the thinnest part of the cornea from the central position, changes in the corneal epithelial layer cell distribution, variations in the anterior corneal astigmatism/posterior corneal astigmatism relationship and a variation in corneal thickness from the central cornea to the periphery with a difference greater than 100 µm requiring further investigation.2,17–20 It therefore follows that any instrument with the ability to detect early signs of KC would be useful in the early diagnosis for better prognostic outcomes. The aim of this review is to identify diagnostic instrumentation capable of detecting pre-clinical KC to enable diagnosis prior to patients reporting subjective symptoms.
The late diagnosis of KC may result in spectacles and contact lenses not being viable treatment options. Collagen crosslinking and ICSR may also be non-viable surgical options as they would have insignificant effect on retarding the disease progression or improving structural deformities. The advanced progression often leads to corneal scarring, making a corneal transplant the only management option. However, the high costs associated with maintaining a cornea donor bank and the cultural dogma linked with tissue donation has resulted in the lack of cornea donor banks in developing countries were they are most needed.21 As a consequence, donor corneas for penetrating keratoplasty have to be imported from the Americas or Europe which makes the procedure very expensive and out of financial reach for the average family in a developing country.21 This in-turn leaves individuals with advanced KC visually impaired with very little hope for improved vision. Identifying a reliable and repeatable method for the early detection of corneal changes will result in earlier diagnosis and management of the condition and subsequently less need for corneal transplants.
Topography is currently the go to assessment in the screening and classification of KC.22–24 Numerous topography indices have been developed to facilitate corneal assessments25 with varied sensitivity and specificity, particularly in the diagnosis of preclinical KC.26–28 An example of such is the cone location and magnitude index whose accuracy in the detection of KC has been verified.27 The Keratoconus predictability index, Belin/Ambrosio enhanced ectasia total derivation value and the Inferior-Superior index have also been shown to be valuable indices in KC investigations.25,26 A combination of wavefront aberometry and higher order aberration video-keratography was shown to be sensitive in distinguishing normal eyes from KC suspects and early KC.29 However, these indices mainly consider anterior cornea assessments whilst pathological changes are evident on the posterior cornea first in ectatic disease.29–31 It is therefore important that we review the corneal imaging methods for the diagnosis of preclinical KC.
MethodAn online literature search was carried out on PubMed, which comprises over 29 million citations for biomedical literature from the United States National Library of Medicine (MEDLINE). The following search terms were used; sub-clinical Keratoconus; topography; tomography; forme fruste Keratoconus and corneal biomechanics. The search was limited to publications between 2010 and 2018 so as to detail the most recent and advanced corneal assessments. Only publications detailing corneal assessment procedures were considered for this review and any publication on instruments that did not generate KC predictability indices were also excluded from the review.
ResultsThe search returned 308 publications.
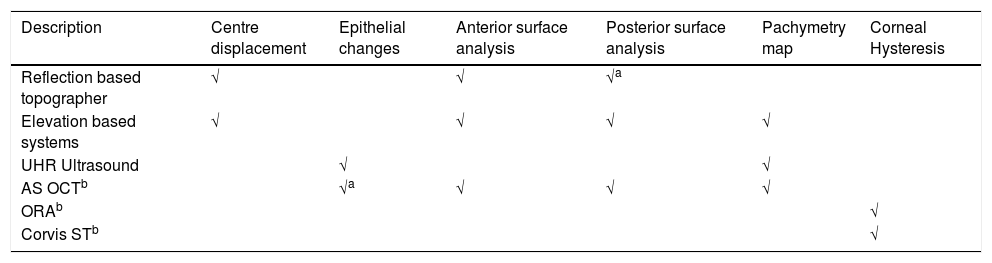
‘Table 1’ shows a summary of the different instruments considered.
Summary of different instrument abilities with regards to corneal analysis investigation.
One of the limitations of this review was the lack of research papers evaluating and validating each individual method of corneal assessment on its own performance in the diagnosis of pre-clinical KC.
Reflection based systemsReflection based systems are the oldest corneal imaging systems on the market with the first computerised topographer manufactured in 1984.32 The placido disc based topography allows for detecting early clinical KC by detecting localised steeping in the anterior cornea surface which is considered to be the first detectable clinical sign of KC33 This may explain why placido disc reflection systems, such as the Medmont, have continued to be referred to as the gold standard in corneal curvature measurements. Weaknesses of the placido disc reflection based systems include the poor repeatability, mainly in the presence of large amounts of astigmatism, and the effects of the skew ray error. However, poor repeatability is often a sign of dry eyes and increased corneal aberrations secondary to the dry eyes which also co-exist with corneal ectasias. The poor repeatability could therefore be used as an indicator for additional investigations.
The multi-coloured LED spot reflection system employed in the Cassini TCA topographer by iOptics was shown to eliminate the problem of repeatability and the skew ray error effects.34,35 The Cassini TCA is the only reflection-based topographer with the ability to assess the posterior corneal astigmatism, giving a measure of total corneal astigmatism. It has been shown to give total corneal astigmatism measures comparable to elevation based systems by assessing both the anterior astigmatism and the posterior astigmatism.36 The most discriminant value for the diagnosis of pre-clinical KC has been shown to be the posterior asphericity asymmetry and the corneal volume.37 Both these values can only be determined by instruments with the ability to assess the posterior corneal surface. The Cassini TCA was shown to produce keratometry, anterior and posterior astigmatism measurements and elevation maps with similar repeatability as a Scheimpflug based system.38
Elevation based systemsElevation based systems either utilise the slit scanning imaging principles, such as in the Orbscan by Bausch and Lomb, or the Scheimpflug imaging principles as in the Pentacam by OCULUS. Combination systems are also available, as in the Galilei G6 by Ziemer, which combines placido reflection with dual Scheimpflug technology and the Orbscan II by Bausch and Lomb which combines placido reflection with slit scanning technology. The ability to take measurements at high speed enables these systems to be more accurate and repeatable than reflection based systems, as they are not affected by eye movements and are able to produce high quality images.20,39
In addition to corneal curvature of both the anterior and posterior surfaces, elevation based systems incorporate algorithms such as the Berlin Ambrosio Display in the Pentacam and the Corneal Objective Risk of Ectasia Screening in the Orbscan.40 These generate indices that describe the posterior asphericity of the cornea and relate it to the anterior corneal surface curvature whilst taking into account other numerical corneal characteristics measured and give a value describing the degree of similarity of the assessed cornea compared to an ectatic cornea. They accurately identify the KC suspect and were proven to be effective in the Asian ethnic group.22,41 These algorithms have revolutionised KC diagnosis and minimise inter-clinician variables as they are well defined. Another useful parameter that the elevation based systems describe is the corneal thickness profile which has been shown to be more sensitive than central corneal thickness alone in diagnosing pre-clinical KC.37
It is also important to note that slit scanning systems and Scheimpflug systems do not generate posterior corneal measurements the same way. Slit scanning systems interpolate the data mathematically whilst Scheimpflug systems actually measure the posterior surface elevation. As the Scheimpflug system measures the true elevation of the posterior corneal surface it can also assess the endothelial layer.19 The Scheimpflug and slit scanning systems are not interchangeable and do not correlate, particularly with posterior corneal measurements but do exhibit better correlation in corneal thickness measurements and anterior corneal curvature.42 Considering all of the related factors, we recommend that elevation based systems be used in conjunction with other assessments such as higher order aberration measuring systems to improve sensitivity and reliability in the diagnosis of pre-clinical KC.
Combination systems such as that seen in the CSO Sirius and the Galilei by Ziemer Ophthalmic Systems which utilise both elevation and reflection based systems are also available and have been shown to be comparable although not interchangeable with elevation based systems.41
Ultra high resolution ultrasoundQualitative assessments of epithelial and stromal thickness maps derived from high resolution ultrasound scans can help distinguish KC from atypical and yet normal corneas. Assessment of the epithelial layer distribution is said to be the only assessment that is 100 % sensitive to pre-clinical KC.18,43,44 The corneal epithelium has five to seven cell layers and a central thickness of approximately 53 µm.44 The cornea has a donut epithelium pattern with a surrounding annulus of thicker epithelium characterised by compensatory thinning over the indicative cone in the presence of corneal ectasia.43,44 This compensatory epithelial cell redistribution allows the stromal changes to go undetected in corneal measurements such as corneal curvature measured by reflection based keratometers or topographers. Epithelial layer thickness has been shown to measure lower in the presence of steep corneal curvature.45,46 With the ability to measure corneal thickness in addition to epithelial layer assessments, Ultra high resolution Ultrasound can differentiate between normal corneas and pre-clinical KC.44 However, it needs to be considered in conjunction with other corneal assessment techniques to conclusively identify pre-clinical KC.
Anterior segment optical coherence tomography (ASOCT)ASOCT utilises faster axial scanning and light of longer wavelength compared to retinal imaging OCT in conjunction with telecentric transverse scanning for corneal imaging.47,48 The Visante OCT by Zeiss which is a time domain system and the RTVue by Optovue which is a spectral domain system are only some of the examples of OCT machines with anterior segment assessment capabilities.48 Ultra high resolution OCT machines such as Bioptigen Envisu by Bioptigen go a step further by detailing finer structures such as the corneal nerves and endothelial layer.49,50 The epithelium ectasia index, Bowman’s layer ectasia index and stroma ectasia index as reported by Ultra High Resolution OCT, such as the MS39 by CSO assess localised thinning vertically with the epithelium ectasia index being the most sensitive for pre-clinical KC.50
IN ASOCT, the thickness of the inferior cornea is compared to that of the superior cornea (I–S), the relative thickness of the thickest corneal section to the thinnest corneal section is reported along with a comparison of the infero-temporal thickness less the supero-nasal thickness (IT-SN) and a value for the thinnest corneal thickness recorded.48 An analysis of these four parameter detects asymmetry which is key in the diagnosis of pre-clinical KC.48
Epithelial layer detailing is the most significant advantage that the ASOCT has over elevation based corneal analysis in the diagnosis of pre-clinical KC.51,52 Epithelium thickness measurements by ASOCT have been shown to be comparable to measurements by high resolution ultrasound.43 ASOCT generates 3D images of the cornea including accurate imaging of the posterior corneal surface.17,50,53 The ability to assess the epithelium, descemet’s membrane and posterior stroma elevation in one examination make ASOCT a superior technique for pre-clinical KC diagnosis, as the combined tests increase sensitivity in screening for the condition. ASOCT has also been shown to be more accurate than elevation based imaging systems in the presence of scarring or corneal haze.54
Corneal biomechanicsThe ocular response analyser (ORA) by Reichert and the Corneal visualisation Scheimpflug technology (Corvis ST) by Oculus are the only commercially available instruments capable of detailing parameters such as corneal hysteresis and formation amplitude that provide an indication of the cornea’s biomechanical robustness. A positive correlation between central corneal thickness and corneal hysteresis has been long reported and it so follows that reduced central corneal thickness equates to compromised corneal hysteresis.55 In addition to reduced corneal hysteresis, a more pronounced deformation profile has been shown by different researchers55,56 with the deformation amplitude being a more sensitive parameter in the diagnosis of pre-clinical KC than corneal hysteresis.55,56 Although deformation amplitude has the most statistically significant difference when comparing normal corneas to ectatic corneas, it still has significant overlap between the two groups.41
A previous study by Scarcelli etal. puts forward evidence suggesting that a focal reduction in biomechanical properties occurs first resulting in tissue thinning as the weaker area strains more than the surrounding healthy areas leading to the development of KC.57 It is unclear as to whether the cornea develops KC due to reduced corneal hysteresis and higher deformation amplitude or corneal hysteresis is reduced as a consequence of the ectasia. A previous study demonstrated that post refractive surgery corneas with reduced central corneal thickness as a consequence of the procedure do have reduced corneal hysteresis.58 This suggests that reduced corneal hysteresis has to pre-exist in ectatic corneas for the condition to manifest. However, further investigation of this is required. It is for this reason we recommend that corneal hysteresis as an indication of biomechanical integrity be considered in conjunction with other corneal assessments in the diagnosis of pre-clinical KC.
ConclusionPre-clinical diagnosis of KC has the benefit of timely management of the condition for improved long-term morbidity outcomes. It enables practitioners to identify the patients that need to be monitored earlier on prior to any clinical symptoms being evident. This will in turn result in more timely interventions being offered when needed. The use of multiple evaluation techniques remains important for a detailed corneal assessment. In addition to topography findings, practitioners should perform additional techniques to be able to diagnose pre-clinical keratoconus for early diagnosis and management of KC.
DisclaimerThe authors have no proprietary or commercial interest in any materials discussed in this article.