To evaluate the 12-month refractive and visual outcomes of Small Incision Guided Human-cornea Treatment (SmartSight®, SCHWIND eye-tech-solutions, Kleinostheim, Germany) in the treatment of myopia corrections with low to moderate astigmatism with the use of a new femtosecond laser system.
Methods221 eyes of 114 patients consecutively treated with SmartSight lenticule extraction were assessed. The mean age of the patients was 28±6 years at the time of treatment with a mean spherical equivalent refraction of -6.26±2.17D and mean astigmatism of 0.92±0.68D. Monocular corrected distance visual acuity (CDVA), uncorrected distance visual acuity (UDVA) were assessed pre- and post-operatively. Refractive changes have been determined in terms of changes in refraction, as well as changes in keratometric readings. The changes in central epithelial thickness have been determined.
ResultsAt twelve months post-operatively, mean UDVA was 20/21±2. Spherical equivalent showed a residual refraction of +0.48±0.31D with refractive astigmatism of 0.13±0.18D postoperatively. There was a slight decrease of -0.1 Snellen lines at 12-months follow-up. The same correction was determined using changes in refraction, as well as changes in keratometric readings. The central epithelial thickness increased by +3±2µm. Spherical equivalent correction within ±0.50D was achieved in 199 eyes (90%), and cylindrical correction in 221 (100%). Preoperative corrected distance visual acuity (CDVA) was 20/20 or better in 213 eyes (96%), and postoperative uncorrected (UDVA) was 20/20 or better in 205 eyes (93%). No eye had lost two or more Snellen lines of CDVA.
ConclusionsMyopic astigmatism correction with SmartSight provided good results for efficacy, safety, predictability, and visual outcomes at the twelve months of follow up. The central epithelial thickness barely increased by 3±2µm.
Lenticule extraction has gained popularity in recent years and has become a serious alternative to LASIK and PRK nowadays.1 Lenticule extraction involves the use of an ultrashort pulse laser system to delineate the contour of a volume of tissue to be excised (providing for the refractive correction) along with a channel to access and extract the lenticule.2
Currently, there is one technology and technique clearly established in the market (SMILE using Visumax by Carl Zeiss Meditec, Germany). Recently, two emerging alternatives have been introduced in the market (CLEAR using Z8 by Ziemer, Switzerland;3 and SmartSight using ATOS by SCHWIND eye-tech-solutions, Germany).4
In particular, for the SCHWIND ATOS and the associated SmartSight treatment, the laser works in the plasma-mediated ablation regime,5 just slightly above the threshold for laser induced optical breakdown,6 and well below the photodisruption regime.7 It uses pulse energies around 100nJ, associated with repetition rates of up to 4MHz, with spot and track spacings of ∼4µm. Further to that, the system incorporates a video based eye registration (cyclotorsion control) from the diagnostic image (to improve the predictability of the astigmatic corrections)8 along with an eye-tracker guided centration.9 The SmartSight profile includes a refractive progressive transition zone10 (similar to the one used in the SCHWIND AMARIS11) tapering the lenticule towards the edge of the transition zone, without the need of a minimum-lenticule-thickness pedestal.12
Since its introduction, Small Incision Lenticule Extraction has received both CE and FDA approval. Small Incision Lenticule Extraction may be associated with less dryness, pain and faster wound healing compared to LASIK (laser assisted in situ keratomileusis) and PRK (photorefractive keratectomy).13
Several factors have been associated with early visual recovery after SMILE, including scanning pattern,14 laser parameters (e.g., spot distance and energy setting),15 and surgical skills.16
This retrospective, observational case series includes 221 eyes of 114 patients who underwent SmartSight to correct myopia with low to moderate astigmatism and completed the 12-month follow-up. Procedures were performed with a SCHWIND ATOS® femtosecond laser (SCHWIND eye-tech-solutions, Kleinostheim, Germany).
MethodsPatientsThis retrospective, observational study comprised eyes that underwent SmartSight for correction of myopia with low to moderate astigmatism at Matrika Eye Center, Kathmandu, Nepal. Before the procedure, patients were adequately informed about the risks and benefits of the surgery. All patients signed informed consent form (ICF) in accordance with the Declaration of Helsinki. The patient demographics and treatment variables are provided in Table 1.
Treatment parameters.
Inclusion criteria included: Subjects 18 years of age or older, able to comprehend and sign an ICF, stable refraction, and discontinuation of the contact lenses prior to the preoperative evaluation. The resulting cohort analyzed in the study had manifest spherical equivalent refractive error ranging from -2 to -13 diopters (D), with up to 3.5 D of astigmatism. Patient charts had CDVA of 20/40 or better, and stable refraction for more than 1 year prior to the study. Patients were required to have normal keratometry and topography, including a calculated central residual stromal thickness of 275µm or more. A total of 221 eyes of 114 consecutive patients were retrieved in the retrospective review chart.
Preoperative examination and treatment planPreoperatively, information on general and ocular medical history, contact lens wear, and medication use was obtained from each patient. The examination included uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, corneal topography/tomography (MS-39; CSO, Firenze, Italy), and slit lamp examination. All tests were performed monocularly. The corrected visual acuity was always assessed with trial frames and not contact lenses.
The sphere and cylinder values entered into the laser were based on the manifest refraction with nomogram adjustments based on the previous experience of the surgeon (these nomogram adjustments evolved during the course of the treatments, and started by adding -0.5D to the manifest sphere and were after some treatments reduced to adding -0.25D to the manifest sphere, but the analyses were performed as deviation from the planned correction, instead of from clinical target). All eyes underwent the refractive treatment using a 6.6- to 7.0-mm lenticular diameter. Re-treatments were not considered for this retrospective study, meaning that that only their visual and refractive data up to the retreatment were included and that of the eyes with completed 12M follow-up, no single eye required a retreatment within that period of time.
Surgical techniqueA single surgeon (KRP) performed the treatments, using an identical surgical protocol. In all cases, automatic cyclotorsion control was verified before the surgery (but no dynamic cyclotorsion control is included after suction). Before the surgery, proparacaine hydrochloride 0.5% drops (Alcaine, Alcon, USA) were instilled 3 times within a 5-min interval. The eyes were opened using a lid speculum. All surgeries were performed with a SCHWIND ATOS femtosecond laser (SCHWIND eye-tech-solutions, Germany). Treatments were planned based on the manifest refraction and performed with non-Wavefront-guided profiles. For centering the treatments, 70% of the corneal vertex offset with respect to the pupil center was used (70% of the distance from pupil to vertex, towards the vertex as obtained from the Sirius tomographer). The used offset (displacement of the optical axis of the lenticule towards the corneal vertex) was implemented by a cross-mark in the computer screen representing the target pupil location (thus leaving the visual axis closely to the optical axis of the system). This is only relevant for cases showing a clearly non-zero pupil offset (somewhat a proxy for the angle kappa, although they are different but related concepts).
After placing the patients on the surgical bed and administering topical anesthesia, patients were instructed to fixate on the fixation light to ensure accurate centration.17 After the patient was positioned on the bed, the cone (a disposable patient interface) was connected to suction ports. The patient's eye was positioned under the cone and patient was instructed to fixate the light target.
In order to accomplish centration and docking of the eye to the system, an eye-tracker guided centration is used. A camera provides the operator with a coaxial view through the cone of the patient interface. The operator is instructed to move the patient table, and bring the pupil (detected by a video-based Eye-Tracker) coincident with the target pupil position (or as close as possible). Then suction was applied at levels between 260mmHg and 290mmHg, and it was confirmed that the pupil remained close to its target position; otherwise a new docking was attempted. Further to that, the last valid laser videoframe of the eye-tracker has been used for cyclotorsion control, and the torsional misalignment from the diagnostic image has been determined and accounted for.21 The treatment was applied and the laser ablation initiated after suction.
For the SmartSight procedure, SCHWIND ATOS works in the low density plasma region,18 just slightly above the threshold for laser induced optical breakdown,19 and well below the photodisruption regime.20 In this series pulse energies between 115nJ and 125nJ have been used with spot and track spacings from 3.7µm to 4.0µm. Caps were 140-150 µm thick, the optical zone ranged from 5.5 to 6.5 mm, the incision was positioned pseudo-superior at 150° with entry angle of 120° and width of 3.0 mm. The optical zone selected depended on the scotopic pupil size and attempted correction. The SmartSight profile includes a refractive progressive transition zone (similar to the one used in the SCHWIND AMARIS) ranging from 0.3mm to 1.0mm (depending on the corneal curvature gradient otherwise induced by the correction22) tapering the lenticule towards the edge of the transition zone, without the need of a minimum-lenticule-thickness pedestal.23 Once the lenticule creation was completed, suction was released automatically. A thin blunt spatula was inserted through the incision to first identify two sides of the lenticule and then separate the lenticule (first the anterior and then the posterior surface) from the stroma and extracted through the incision. After extraction, cornea was gently massaged (ironed) in straight movements from the 6 o'clock position towards the incision in order to spread the cap evenly and potentially decrease Bowman's wrinkles.24 In the end the remaining tissue was checked for any residual material or tears.
Postoperative examinationsFor this cohort, patients were instructed to visit the clinic for postoperative examinations after 12 months. Postoperative examinations included UDVA, CDVA, manifest refraction, corneal topography/tomography (MS-39; CSO, Italy), and slit lamp examination. Only patients with completed follow-up were included in the review chart.
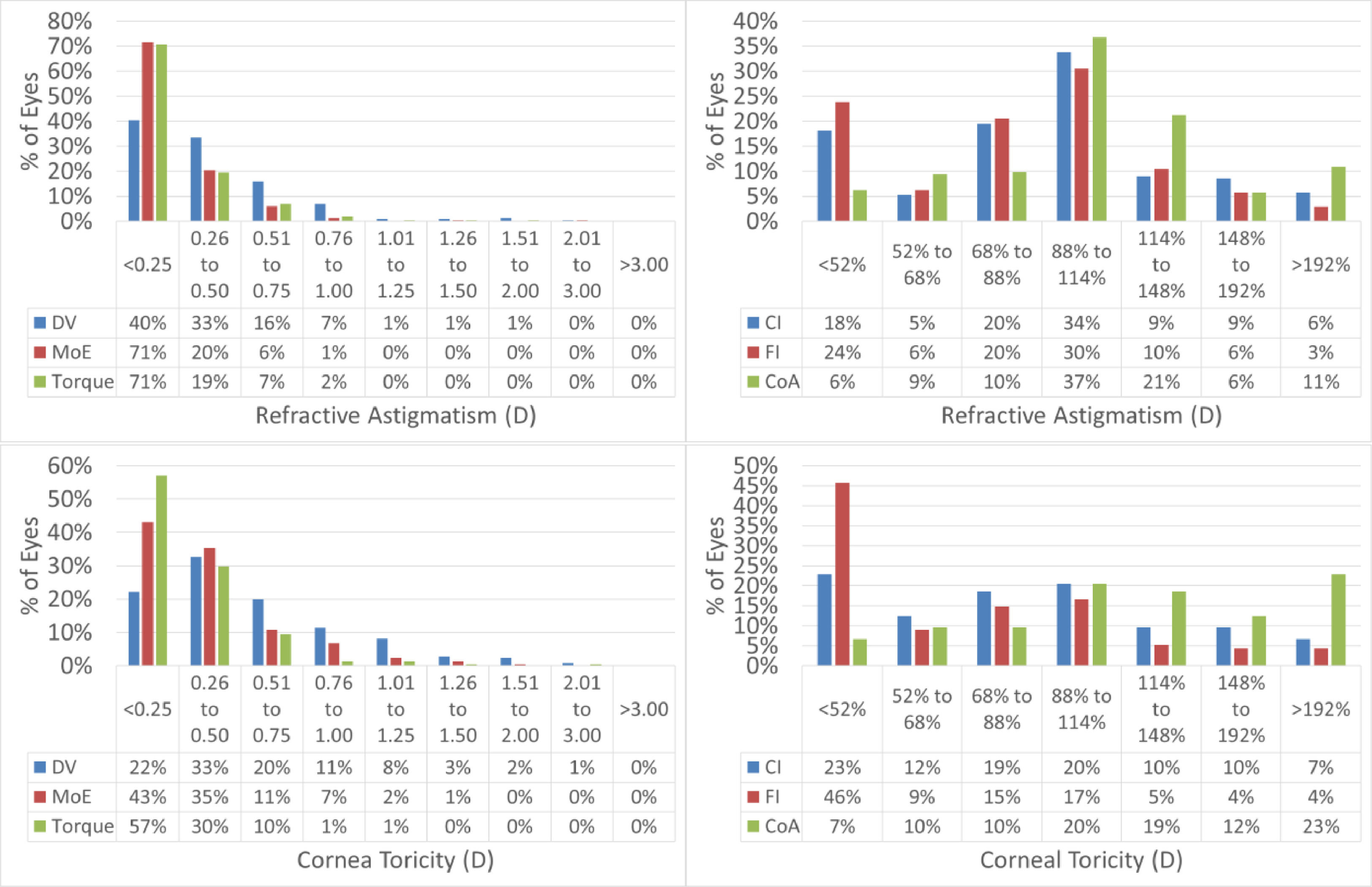
Vector analysisVector analysis as described by Alpins25 has been performed. This includes the evaluation of Target Induced Astigmatism (i.e. the planned astigmatism), Surgically Induced Astigmatism (i.e. the achieved change in refractive astigmatism), Correction index (the ratio surgically induced to target induced astigmatism), Magnitude of Error (i.e. the difference between the magnitudes of surgically induced and target induced astigmatism), Angle of Error (i.e. the angular displacement between the surgically induced and the target induced astigmatism), Difference Vector (i.e. the magnitude of the vectorial difference between surgically induced and target induced astigmatism), Flattening Effect (i.e. the magnitude of flattening achieved at the axis of the target induced astigmatism), Flattening Index (i.e. the ratio flattening effect to target induced astigmatism), Torque (i.e. the magnitude of astigmatic correction achieved at a 45deg axis from the target induced astigmatism), and Coefficient of Adjustment (i.e. the reciprocal of the correction index).
Statistical analysisAnalysis of variance and t-tests were performed between preoperative and post-operative status. Visual acuity was measured in Snellen equivalent fraction, converted to logMAR for analyses, and reported back in Snellen equivalent fractions for comparability. Uncorrected and corrected visual acuity, spherical equivalent refraction, refractive astigmatism, cardinal and oblique astigmatism, surgically induced astigmatism, and angle of error were analyzed. The Coefficient of Determination (r2) was also employed and the significance of the correlations has been evaluated assuming a metric that is distributed approximately as t with N—2 degrees of freedom, where N is the size of the sample
A level of significance at P < 0.05 was used for all statistical tests. Due to correlation between left and right eye, statistics have been performed considering the number of patients and not number of eyes. Statistical analyses have been performed using Excel (Microsoft, USA), and the same was used for generating the figures.
ResultsOf the complete cohort, 114 patients completed the 12-months follow-up and were included for analyses. A summary of the preoperative and postoperative demographics is presented in Table 2.
Preoperative and postoperative outcomes.
All patients had a remaining corneal residual bed thickness of 300µm or more. A complete dissection and removal of the lenticule was achieved in all cases without relevant intraoperative complications, and at postoperative day 1, all patients had a clear cornea.
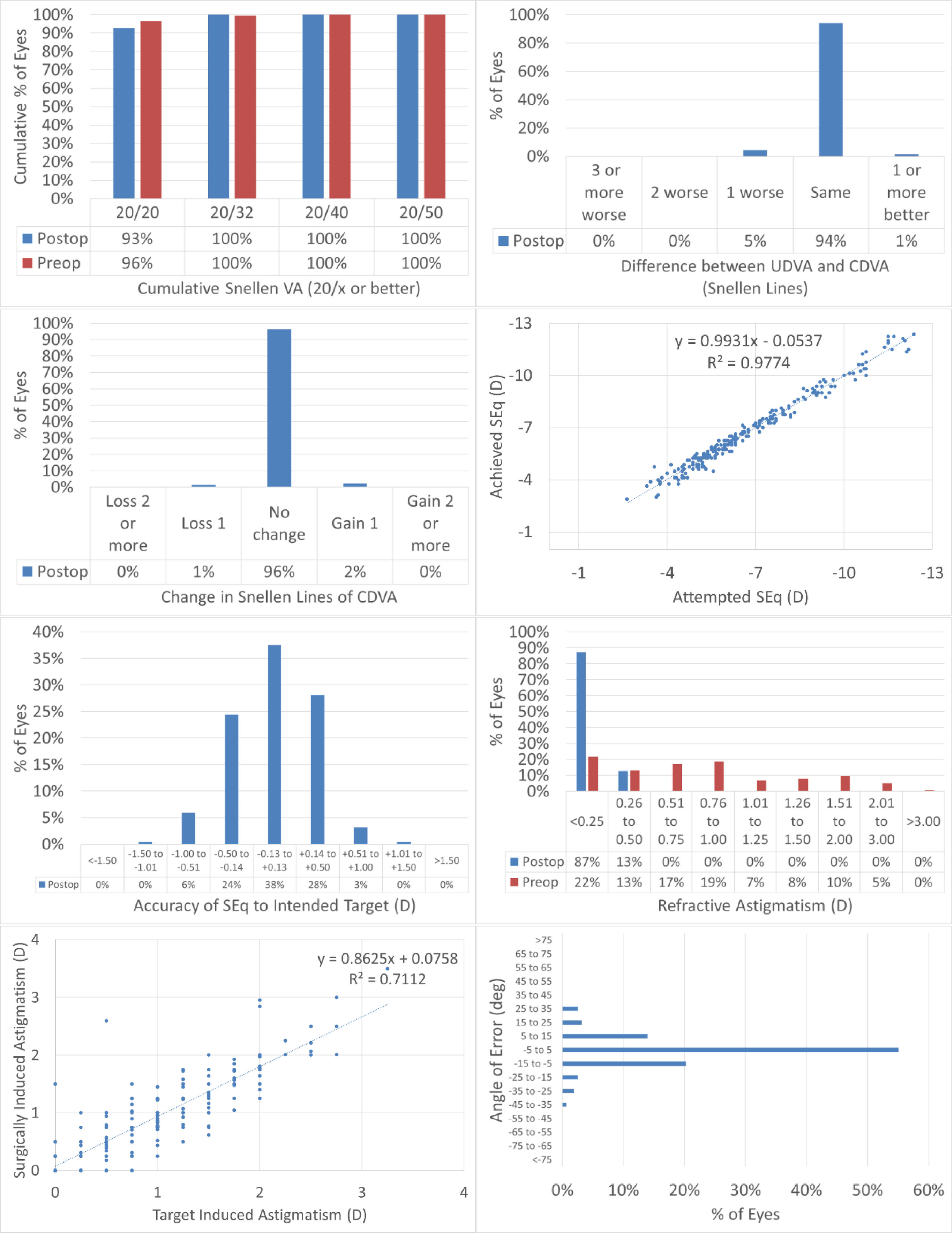
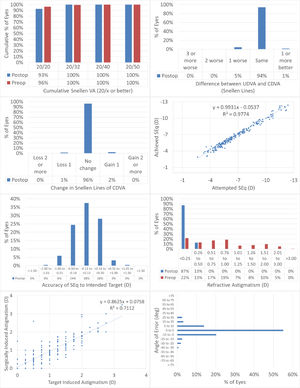
Fig. 1 shows the results in standardized graphs and terms for refractive surgery outcomes.26 The outcomes at the 12-months postoperative follow-up visit are presented and compared to the preoperative status.
The results of performing SmartSight for the treatment of myopic corrections with no to moderate astigmatism with the use of SCHWIND ATOS, presented graphically using the standardized graphs and terms for refractive surgery results. The refractive outcomes were evaluated for changes in manifest refraction for the whole cohort.
At 12M, 93% of the eyes reached an UDVA of 20/20 or better (Fig. 1A), for all eyes UDVA remained within one line of preoperative CDVA (Fig. 1B), no eyes lost 2 lines of CDVA (Fig. 1C). At 12M, the scattergram showed an excellent refractive correction of the SEQ and refractive astigmatism (Fig. 1D and 1G), with 90% and 100% of the eyes within 0.5D from target for SEQ and refractive astigmatism, respectively (Fig. 1E and F). for 89% of the treatments the axis of the refractive astigmatism was within 15deg from the plan (Fig. 1H).
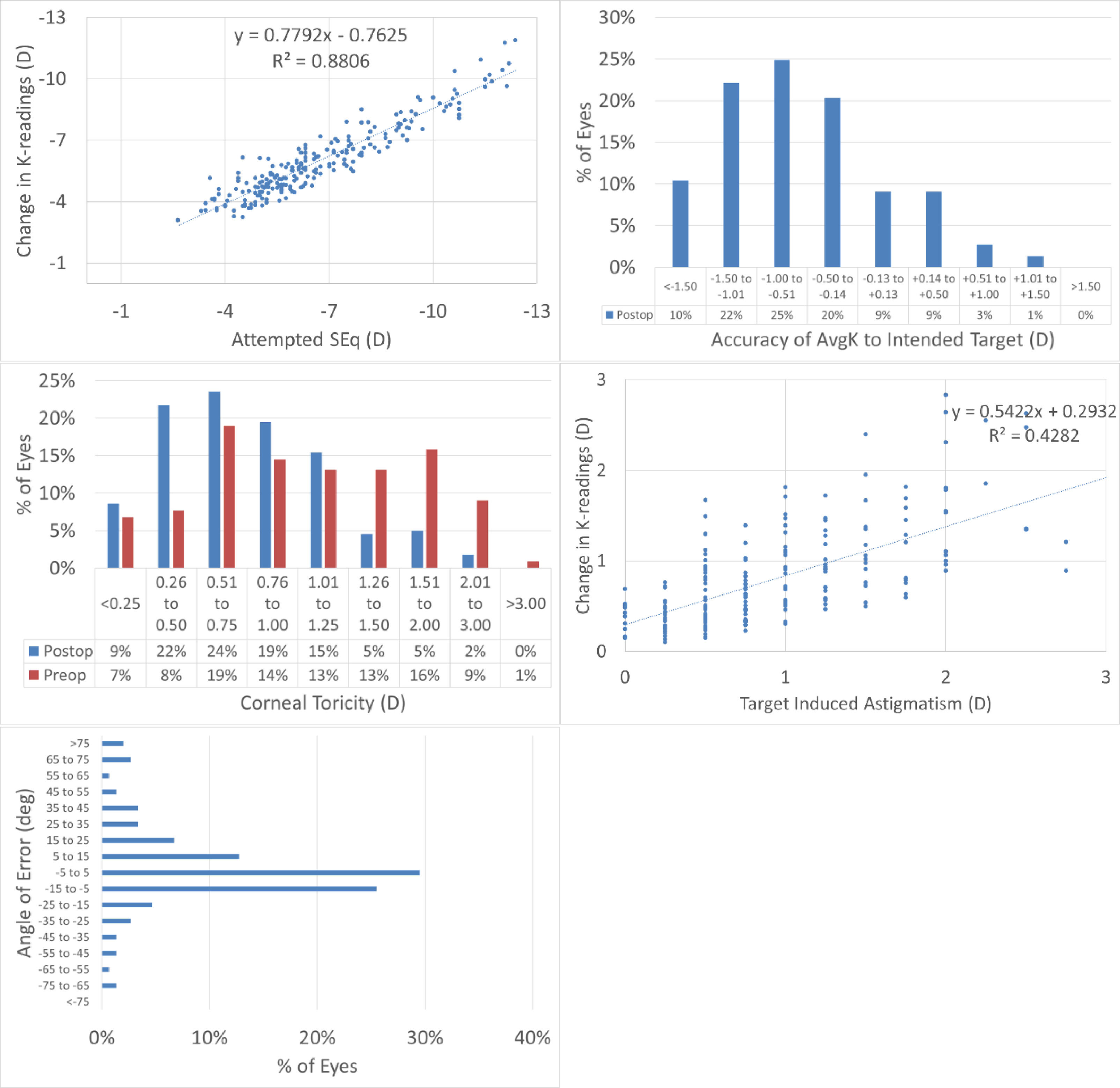
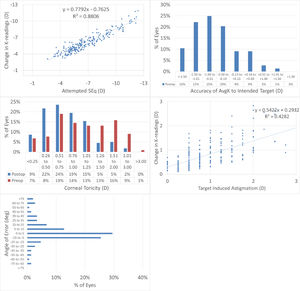
Topographic changesAt 12M, the scattergram of achieved change in keratometry readings vs. attempted refractive correction of the SEQ showed a very good correlation (Fig. 2A), with 65% eyes within 1D from target (Fig. 2B). The scattergram of achieved change in keratometry readings vs. attempted refractive correction of the astigmatism showed only a low correlation (Fig. 2C), with 73% eyes with postoperative corneal toricity of 1D or less (Fig. 2D). The angle of error was within 25deg from attempted astigmatism axis in 79% of the eyes (Fig. 2E).
The results of performing SmartSight for the treatment of myopic corrections with no to moderate astigmatism with the use of SCHWIND ATOS, presented graphically using the standardized graphs and terms for refractive surgery results. The refractive outcomes were evaluated for changes in corneal keratometries for the whole cohort.
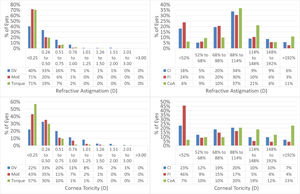
Vector analysis is displayed in Fig. 3 and presented in Table 3.
Vector analysis of the astigmatism outcomes.
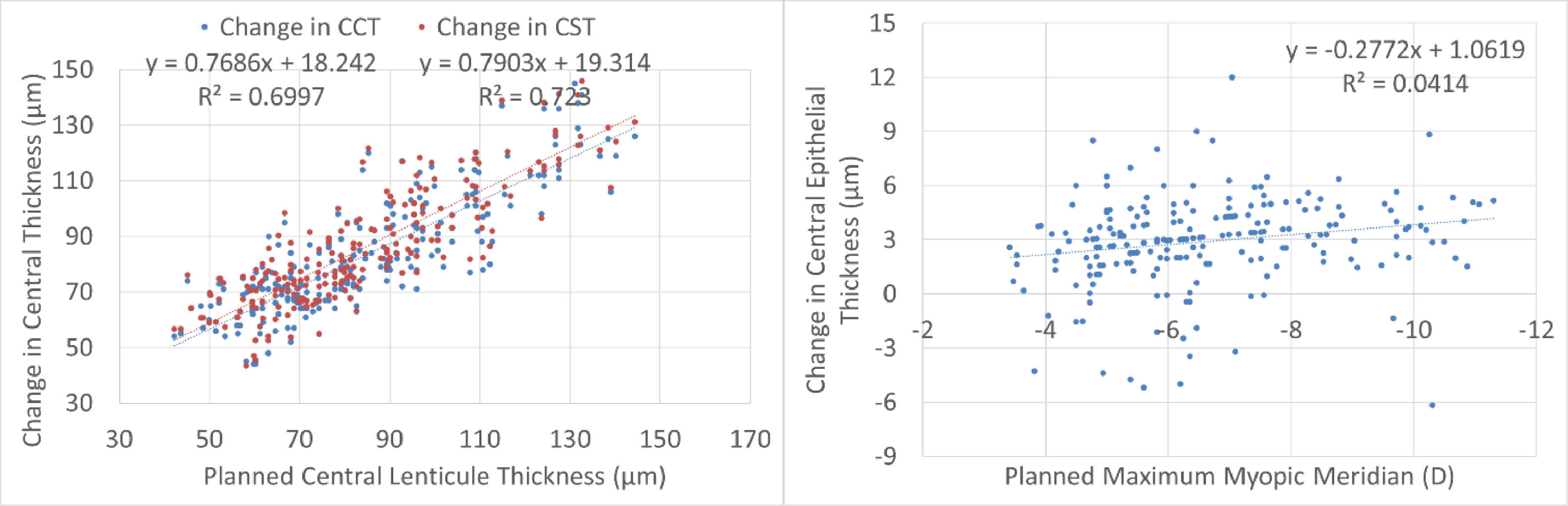
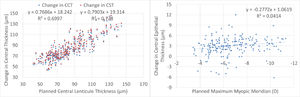
Central thickness analysis is displayed in Fig. 4. There was a very good correspondence between planned lenticule thickness and the reduction in central pachymetry or central stromal thickness. There was no significant correlation between the planned correction and the difference in central epithelial thickness. The central epithelial thickness increased on average by +3±2µm but ranged from -6µm thinner to +12µm thicker.
ComplicationsObserved complications have been recorded, including suction loss, incisional bleeding, subconjunctival hemorrhage, tearing of the lenticule, and abrasion at the incision for which no single event has been observed.
Opaque bubble layer occurred in 57 eyes (26% of the treatments), whereas black spots occurred to some extent in 19 eyes (9% of the treatments), and areas or inaccurate laser pulse placement due to eye movement in 2 eyes (1% of the treatments). In 22 cases (10% of the treatments) initially the posterior plane was entered, this was detected by the surgeon and the anterior plane was subsequently entered.
DiscussionThis cohort study was based on a consecutive case series of patients treated by a single surgeon (KRP), with SmartSight to correct myopia (with or without refractive astigmatism), at Matrika Eye Center in Kathmandu, Nepal.
In this study, for all the patients, lenticule removal was complete without relevant intraoperative complications. There were no events of suction loss in this series (though suction loss occurred before the treatment started in 2 lenticule attempts and 2 further flap attempts out of these series), no cases of incisional bleeding or subconjunctival hemorrhage have been recorded, no tearing of the lenticule has been observed, as well as no abrasion at the incision.
Concerning opaque bubble layer and black spots/areas or inaccurate laser pulse placement due to eye movement, while they were observed at times (with an incidence slightly higher from what has been reported in the literature,27 this may due to the fact that for both the posterior and the anterior cuts, the laser scan is centrifugal, i.e. starting from the centre and moving outwards), they have not been considered a relevant complication for at least two reasons. On one hand, they did not interfere with the dissection (subjectively there was no impression that more resistance during dissection was found for those eyes), and on the other hand (unlike for flaps) there is no subsequent treatment step (excimer laser) for which the presence of opaque bubble layer may interfere with the tracking system (of the excimer laser).
In a previous study,28 the authors reported in vivo morphology of opaque bubble layers with ultrahigh-resolution anterior-segment optical coherence tomography (UHR-OCT) in 3 patients. Two patients were operated on with a 30-kHz IntraLase femtosecond laser (Abbott Medical Optics, Abbott Park, IL) and one patient was operated on with a 500-kHz VisuMax femtosecond laser (Carl Zeiss Meditec, Jena, Germany). UHR-OCT images from the patient operated on with the 500-kHz femtosecond laser revealed that the opaque bubble layer extended anterior to the flap dissection plane up to Bowman's membrane. The lamellar flap dissection was incomplete in this patient. The opaque bubble layer in the patients operated on with the 30-kHz femtosecond laser extended posterior to the flap dissection plane and these patients experienced complete lamellar dissections with uncomplicated flap lifts. It is possible that the opaque bubble layer induced by ATOS is also posterior to the flap dissection plane and thus they did not interfere with the dissection.
As for black spots, in our experience black spots were observed as isolated punctual micro-regions, and they seemed more related to debris at the cornea-laser interface than to instability of the laser energy.29 We think that one reason why they did not interfere with the dissection can be the fact that ATOS is using a relatively high Repetition Rate, in the MHz regime, associated with a low energy per pulse with dense overlap.30 Thus, many tiny microbubbles are created close together; probably stochastically homogenizing the ease of dissection.
SCHWIND ATOS works slightly above the threshold for laser induced optical breakdown,31 in the plasma-mediated ablation regime,32 generating only low-density plasma,33 and well below the photodisruption regime. In this series pulse energies between 115nJ and 125nJ have been used with spot/track spacings from 3.7µm to 4.0µm.
Unintended initial posterior plane dissection never occurred completely, i.e. in the cases for which initially the posterior plane was entered, this was detected by the surgeon and the anterior plane was subsequently entered.
After extraction, the cornea was gently massaged (ironed) in straight movements from the 6 o¨’clock position towards the side cut incision in order to spread the cap evenly and potentially decrease Bowman¨’s wrinkles.34
With Visumax, usually a residual stromal thickness below the cap of at least 250µm is advised, whereas 275µm are used as the limit with the SCHWIND ATOS. The limit of 250µm for lamellar refractive surgery35 (whether LASIK or lenticule extraction) is no longer adopted in many regions, and clinics. It seems more that 280-300µm is more common nowadays.36 275µm ist „just“ +10% above 250µm as a safety margin, the 25µm extra safety margin can be understood as a reserve for potential undercorrections of up to ∼1.5D; but at the same time is ∼50% (half the thickness) of the average normal central corneal pachymetry;37 finally, since the introduction of percentage tissue altered (PTA), a PTA of ∼40% has been proposed as the cutoff value for enhanced ectasia risk,38 considering 480µm (according to the german “Kommission Refraktive Chirurgie der DOG und des BVA”)39 as the minimum pachymetry suitable for lamellar refractive surgery (whether LASIK or lenticule extraction); an RST of 275µm would represent a 42.7% PTA.
Strictly speaking, PTA may only apply only to LASIK, or if e.g. a circle treatment (or any other type of lenticule extraction to LASIK conversion) is performed after a lenticule extraction for a refractive enhancement. Otherwise, the PTA concept does not apply directly to lenticule extraction by only considering the RSB as preserved tissue, since the cap thickness may not count (in full) for the PTA as "severed tissue" (at least with current evidence).
The calculated residual central corneal thickness was set to 275µm or more, and no single eye has actually a calculated residual central corneal thickness below 300µm. The used cap parameters with ∼8mm diameter and 143µm thickness were in the normal range used in other lenticule extraction procedures. Although, the literature is controversial as to whether thicker caps result in biomechanically stronger40 or weaker corneas.41 Some recent publications even suggest a nomogram compensation based on the planned cap thickness.42
Caps were 140-150 µm thick,43 the optical zone ranged from 5.5 to 6.5 mm,44 the incision was positioned pseudo-superior at 150° with entry angle of 120° and width of 3.0 mm.45
Corneas in Nepal are rather small, and so are pupil sizes as well, thus a smaller OZ (compared to other regions in the world) is usually adopted. Further, treatments were in general of moderate to high myopia (average myopic meridian of -6.73D) which are associated with smaller planned OZ as a measure to reduce tissue consumption (and respect RST of 275µm).
In our cohort, we evaluated short-term refractive and visual outcomes after SmartSight for the treatment of myopic corrections with no to moderate astigmatism with the use of SCHWIND ATOS.46 Lenticule extraction approach with the Visumax SMILE has proved its value in myopic corrections.47 Nevertheless, stability of the refractive error might be an issue, thus we report here the 12-month outcomes.
For 7 patients only one eye was included in the retrospective review chart. For them, the refraction in the excluded eye would result in a lenticule of less than 40µm, and for those eyes no lenticule extraction has been attempted so far (they have been treated using transepithelial PRK).
Preoperatively, the patients had a CDVA below the normal (mean 20/20±2 with range to 20/40). This CDVA could be a characteristic specific to our patient population. In our cohort no eyes lost 2 Snellen lines of CDVA at 12-months postoperatively. At the 12-month follow-up 93% of the eyes had a UDVA of 20/20 or better, without losses of CDVA. This is comparable to current LASIK and SMILE reports.
The scattergram of attempted versus achieved SEQ indicates a correction close to perfect (+0.05D overcorrection), whereas the correlation between TIA and SIA showed a slight undercorrection. Vector analysis further confirms the good astigmatic correction in this series.
Considering the vector analysis of astigmatism, the change in manifest refractive astigmatism correlated well with the attempted values, suggesting that laser surgery could modify the corneal contour properly in moderately toric eyes, as well. Therefore, it can be inferred that the planned goal of the treatment was met, even if patients were slightly undercorrected for refractive astigmatism.
This case series analyzed the 12M follow-up of the efficacy and safety of lenticule extraction treatments using the SmartSight profile. This technique aims to compete in the lenticule extraction. The analysis revealed promising results after the treatment. Unaided vision was expected to improve overall. Most of the outcome measures showed significant improvement compared to the preoperative status.
At 12M, 93% of the eyes reached an UDVA of 20/20 or better (Fig. 1A), for all eyes UDVA remained within one line of preoperative CDVA (Fig. 1B), no eye lost 2 lines of CDVA (Fig. 1C). At 12M, the scattergram showed an excellent refractive correction of the SEQ (Fig. 1D), with 90% of the eyes within 0.5D from target (Fig. 1E).
An excellent refractive outcome was observed in terms of manifest refraction, but this was only partly confirmed by the topographical changes. This suggests that manifest refraction may be more forgiving in terms of exactly determining the accuracy of the treatments;48 but at the same time UDVA is a main driver for patient satisfaction.
At 12M, for the change in corneal keratometry 66% eyes were within 1D from target (Fig. 2B), with 73% eyes within 1D corneal toricity (Fig. 2D). The angle of error was within 25deg from attempted astigmatism axis in 79% of the eyes for corneal keratometry (Fig. 2E).
Keratometries were measured by Placido-OCT-based corneal topography (MS-39) by averaging three consecutive examinations. This average may already provide a “low pass” filter in the analyses and shall better represent the likely “true” keratometries (pre and post).
In any refractive procedure, an ideal feature would be correction efficiency irrespective of the preoperative curvature of the cornea of the patient. Many studies have shown an increase in the correction efficiency for steeper corneas in myopia correction.49 Similarly, another ideal feature would be that the correction measured in the cornea (using adequate models) would correspond 1-to-1 to the correction measured in the refraction, enabling a direct dose response control on an objective manner.50
Munnerlyn et al, in their original paper, already expressed that the attempted correction univocally determines the postoperative corneal curvature (corneal refractive power).51 Conversely, it should be possible to objectively determine the refractive correction by comparing the postoperative with the preoperative corneal curvature (corneal refractive power).
It has been reported that total corneal refractive power measurement (TCRP) may provide more accurate evaluations of the central corneal power compared to simulated keratometry and true net power method.52
In previous publications it has been suggested that the undercorrection in SMILE may occur in high refractive treatments and could be associated with changes (steepening) of the posterior curvature as a result of a forward shift of the posterior surface. This has been reported in a recent study by Sideroudi et al.53 It has also been supported by the results presented by Ganesh et al.54
Other works report that the change observed in keratometries is lower than the change observed in the refraction, in our opinion this is partly related to the fact that these works used oversimplified models ignoring effects like the different refractive indices used for keratometry and actual refractive index of the cornea, or the effects of the vertex distance (from spectacle plane to the corneal plane) on the planned refractions, or the effect of central tissue removal on the refraction (shortening the axial length).
The SmartSight profile includes a refractive progressive transition zone (similar to the one used in the SCHWIND AMARIS) ranging from 0.3mm to 1.0mm (depending on the corneal curvature gradient otherwise induced by the correction) tapering the lenticule towards the edge of the transition zone,55 without the need of a minimum-lenticule-thickness pedestal.
This may be one of the reasons for the minimum changes observed in central epithelial thickness. In turn, the reduction in central corneal thickness and central stromal thickness were very similar and correlated very good to the planned lenticule thickness. This also confirms the lack of undercorrection, underablation, or undersizing of the lenticule in this series.
A recent work56 evaluated the postoperative behavior of the central corneal stroma thickness after myopic femto-LASIK and SMILE by using a combined anterior segment-OCT and placido disc topographer, and compared the accuracy of both laser machines in predicting the real stromal change. After LASIK, the stroma showed a significant rethickening between months 1-3 (+4µm at the centre; p < 0.001), remaining stable thereafter. After SMILE, the stromal thickness remained stable from 1-month. Stromal ablation prediction was higher for SMILE compared to LASIK for all SE ranges, although postoperatively such differences were significant only for ametropias≤4D. At 6 months, mean SMILE laser prediction error was -13±7µm, while LASIK prediction showed better accuracy (+1±8µm; p<0.001). In our cohort, we did not track the longitudinal changes of the stromal thickness, but at 12-month follow-up, the mean SmartSight laser prediction error was +2±12µm (much less than what has been reported for SMILE, and very close to what has been reported for AMARIS).
Lenticule extraction has gained popularity in recent years,57 and has become a serious alternative to LASIK and PRK nowadays.58 Currently, three technologies compete in this market (SMILE using Visumax by Carl Zeiss Meditec, Germany;59 CLEAR using Z8 by Ziemer, Switzerland; and SmartSight using ATOS by SCHWIND eye-tech-solutions, Germany).60
A limitation of this work is the retrospective nature of the study. A number of confounding factors may be argued in our review, we have considered both eyes of the patients. The average age at the time of treatment was 28±6 years (19 to 54 years; median age was 27years), this may be inline with current trends in LVC, but this means that these findings may not hold true for older patients.
The ATOS system incorporates a video based eye registration (cyclotorsion control) from the diagnostic image (to improve the predictability of the astigmatic corrections)62 along with an eye-tracker guided centration.63 For centering the treatments, 70% of the corneal vertex offset with respect to the pupil center was used (70% of the distance from pupil to vertex, toward the vertex as obtained from the Sirius tomographer; CSO). We aimed to have the treatment centred more prone towards the corneal vertex (as a proxy for the visual axis) than towards the pupil centre (equivalent to the line-of-sight in the presence of proper fixation). In a LASIK study, it has been shown that 80% of the offset (towards the corneal vertex) was providing better outcomes than 50% and 100% of the offset.61 Further to that, the last valid laser videoframe of the eye-tracker has been used for cyclotorsion control, and the torsional misalignment from the diagnostic image has been determined and accounted for.
The presented clinical outcomes are based on 12-months of clinical follow-up, which is considered the a moderate long-term meaningful in refractive surgery. However, shorter follow-ups have been reported in the literature to determine the time-course of visual recovery. Longer follow-ups could shed light on the durability of performance.
In conclusion, we demonstrated that myopic corrections with no to moderate astigmatism correction with SCHWIND ATOS SmartSight provided good results in terms of efficacy, safety, predictability, and visual outcomes at the 12-month follow-up.
Financial supportNone.
Ethics statementEthics approval was not required due to the retrospective nature of the review chart. The purpose of this clinical research does not represent a clinical investigation. The medical device was used within its intended purpose without any additional invasive or patient burdensome procedures used.
SYNOPSIS:
The 12-month outcomes of SmartSight for the treatment of myopic corrections with no to moderate astigmatism in 221eyes with the SCHWIND ATOS showed excellent levels of efficacy, safety, predictability, and stability for up to 3D of preoperative refractive astigmatism. The changes in central epithelial thickness were minimum at 12-month postoperatively.