Visual cues usually play a vital role in social interaction. As well as being the primary cue for identifying other people, visual cues also provide crucial non-verbal social information via both facial expressions and body language. One consequence of vision loss is the need to rely on non-visual cues during social interaction. Although verbal cues can carry a significant amount of information, this information is often not available to an untrained listener. Here, we review the current literature examining potential ways that the loss of social information due to vision loss might impact social functioning. A large number of studies suggest that low vision and blindness is a risk factor for anxiety and depression. This relationship has been attributed to multiple factors, including anxiety about disease progression, and impairments to quality of life that include difficulties reading, and a lack of access to work and social activities. However, our review suggests a potential additional contributing factor to reduced quality of life that has been hitherto overlooked: blindness may make it more difficult to effectively engage in social interactions, due to a loss of visual information. The current literature suggests it might be worth considering training in voice discrimination and/or recognition when carrying out rehabilitative training in late blind individuals.
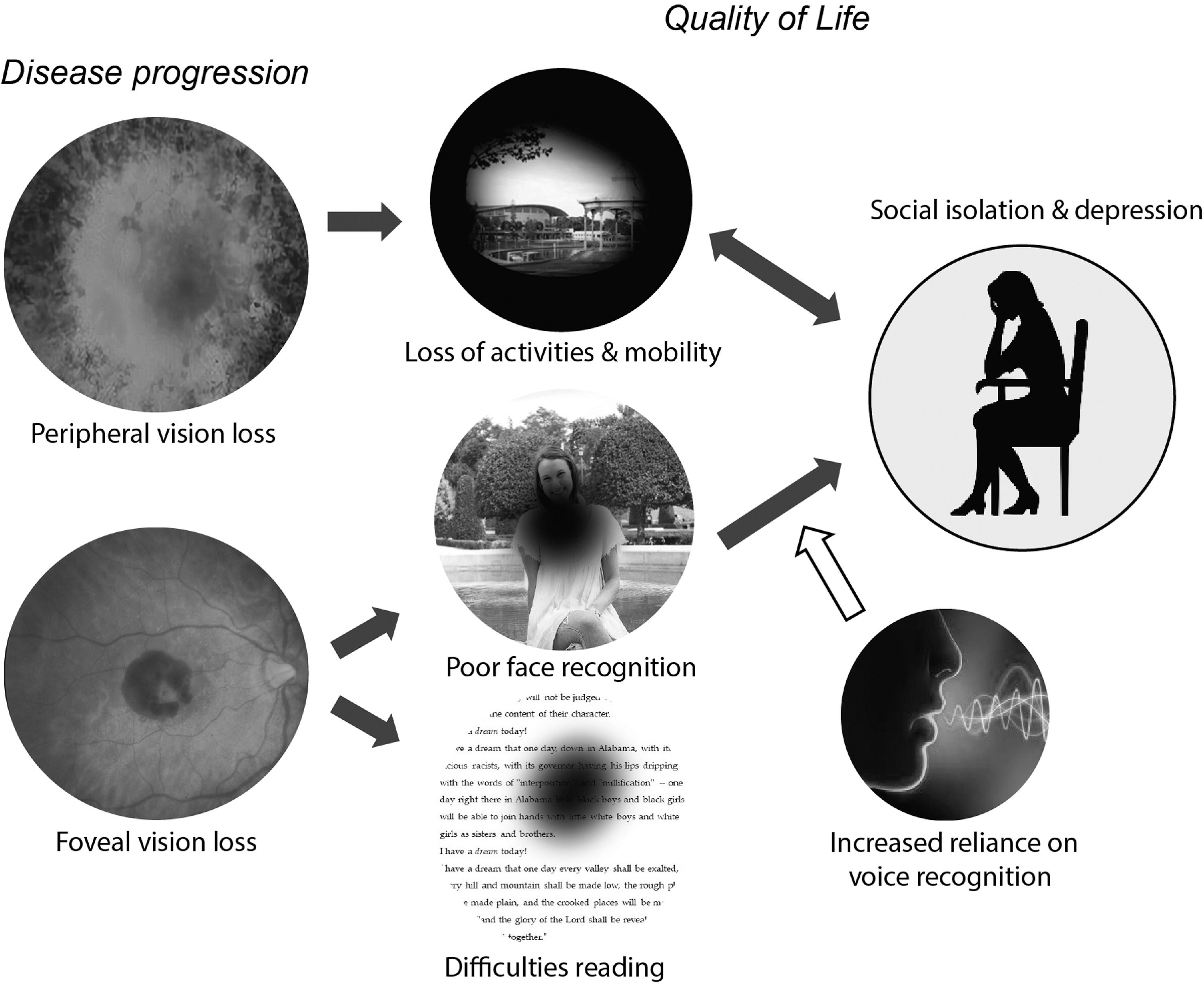
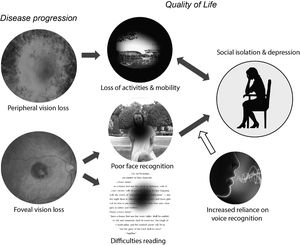
Low vision is associated with a reduced quality of life, defined as a “complex trait that encompasses vision functioning, symptoms, emotional well-being, social relationships, concerns, and convenience as they are affected by vision”.1 Vision loss can reduce quality of life in a wide variety of ways, including difficulties in reading, restrictions in activities and employment, and limitations in both physical and practical (e.g. driving a car) mobility. As a consequence, it is easy for vision loss to result in reduced engagement in social and pleasurable activities. The effects of vision loss on quality of life are complex and multifaceted, Fig. 1; In this review we focus on one single aspect of quality of life that has not been explicitly examined - the potential causal influence of poor face recognition on social isolation, anxiety and depression.
It is now well established that low vision and blindness are risk factors for both anxiety and depression.2,3 However, the relationship between loss of vision and these psychosocial impacts is not yet fully understood.
Current models of depression and anxiety emphasize their strong comorbidity. Up to two-thirds of adults with anxiety disorders may also suffer from depression.4 Anxiety and depression share clinical symptoms as well as social, psychological, neurobiological and genetic and risk mechanisms.5
As far as psychological and social risk factors are concerned, it is believed that anhedonia (a reduced ability to feel pleasure) and the withdrawal from pleasurable activities may be a major contributing component for both anxiety and depression. The relationship is thought to be bidirectional: depression leads to a withdrawal from pleasurable and/or social activities, and the loss of pleasurable activities and social contact worsens depressive or anxiety symptoms.6–8
Consistent with this model, it has long been recognized that the susceptibility to anxiety and depression observed, based on meta-studies, in almost every low vision and blind population9 may be at least partially driven by a lack of access to pleasurable and social activities. The practical impacts of vision loss on quality of life and social function are well recognized. For example, individuals with peripheral field loss can no longer drive safely, which can easily lead to social isolation.10,11 Individuals with central field loss which impacts reading are likely to have restricted employment opportunities.12 This can be exacerbated by worry about disease progression, which contributes to feelings of frustration, fear, and sadness.13,14
However, another more subtle potential cause of anxiety and depression in blind individuals, that has hitherto been unexplored, may be the loss of important non-verbal social information, such as facial expression and body language.
Schematic model of potential relationships between vision loss and reduced quality of life. Vision loss can reduce quality of life in a wide variety of ways, including restrictions in both physical and practical (e.g. driving a car) mobility and difficulties reading. The loss of pleasurable activities and social contact worsens depressive or anxiety symptoms. The relationship is bidirectional: depression can lead to a further withdrawal from pleasurable and/or social activities. In this review we focus on the need for blind and vision impaired individuals to rely more heavily on non‐visual information to recognize individuals and understand their emotions. The resulting difficulties in social processing may provide a causal route to social anxiety and depression.
Facial cues clearly have deep ecological importantance. Processing of these cues is associated with a wide network of subcortical and cortical areas that include the putamen and the cerebellum as well as visual, limbic, temporoparietal and prefrontal cortices.15 Difficulties in recognizing individuals or recognizing emotions makes social functioning more difficult across a wide range of disorders, including prosopagnosia and Autism Spectrum Disorder (ASD),16–20 as described in more detail below. Thus, it seems plausible that low vision that impairs face processing may be a hitherto unexplored additional risk factor for depression, social isolation and anxiety.
In this review, we begin with a selective review of early and late onset blindness as a risk factor for social anxiety and depression. Although quality of life assessments have clearly demonstrated the impact of blindness on social and mental health, and multiple potential contributing factors have been identified, the causal pathways are still not well understood, and are likely to differ as a function of the type of vision loss. Next, we discuss a potential relationship between difficulties in understanding visual facial information and social anxiety and depression in individuals with prosopagnosia and ASD. Finally, we discuss evidence that training might improve voice recognition abilities, and thereby lessen social difficulties due to face recognition loss.
Psychosocial consequences of early onset low visionIt is now well established that early vision loss has psychosocial consequences. Children with retinopathy of prematurity (ROP, bilateral abnormal vessel growth and subsequent retinal detachment due to prematurity) perform significantly worse across multiple subscales of the Children's Visual Function Questionnaire (CVFQ),21,22 with increasing severity of ROP linked to worsening overall quality of life.21 Consistent with our hypothesis that impairments in face recognition has social consequences during development, children with ROP had lower CVFQ personality scores (e.g. “My child likes to visit with relatives.”, “My child gets along well with our other children and friends”, “My child makes new friends easily”, “My child enjoys playing with others (sisters and brothers or friends).”).
Psychosocial well-being scores are similarly reduced in children with microphthalmia (ocular malformations in newborns resulting in smaller than average eyes), anophthalmia (the eyes fail to develop prenatally) and coloboma (ocular malformation affecting the eyelid, lens, macula, optic nerve, choroid, iris, or corpus ciliare),23 as well as in children with retinoblastoma (an ocular cancer that can require removal of the eye).24
At least some of these psychosocial impairments may persist into adolescence. One small study using congenitally blind and sighted matched adolescents found that depression and self-concept characteristics of adolescents with visual impairments were similar to those of sighted adolescents; however the anxiety levels of adolescents with visual impairments were significantly higher than those of their sighted peers.25
Psychosocial consequences of late onset low visionVisual impairment is associated with reduced social participation, with a recent meta-review finding a strong consensus in the literature that visual impairments results in a withdrawal from social activities. There was also some indication that participation in group based activities (e.g. clubs/associations) was more heavily impacted than the quality of relationships with friends or family.26
Depression and anxiety is also common in individuals with late onset visual loss, regardless of whether foveal or peripheral vision is primarily affected. Although the association between visual loss and anxiety is not as clear as the association between visual loss and depression, anxiety is a significant symptom in many individuals.27 Consistent with a model where depressive symptoms result in social isolation, which in turn worsen depressive symptoms, agoraphobia and social phobia are the most prevalent anxiety disorders in visually impaired older adults.3
Much less is known about whether psychosocial impairments vary with the type of vision loss. Unfortunately, most studies have focused on a particular type of vision loss, and have rarely clearly differentiated difficulties in social functioning, generalized anxiety and depression. One exception is the Michigan Vision-related Anxiety Questionnaire, designed to measure psychosocial outcomes across a variety of inherited retinal degenerations. This work provides one of the few attempts to differentiate different forms of psychosocial impairment based on the form of vision loss. This questionnaire revealed two domains of anxiety in patients with inherited retinal degenerations: cone dysfunction and rod dysfunction related anxiety. Only central vision loss due to cone dysfunction resulted in worries about recognizing faces.28
Age related macular degeneration (AMD)In the earlier stages of AMD (a progressive degenerative disease of the macula, the central area in the retina responsible for the high visual acuity required for reading and face recognition) symptoms include difficulty seeing objects clearly and/or apparent distortions. Over time, without treatment, vision slowly deteriorates, resulting in the loss of significant regions of central vision.
Unsurprisingly, AMD has been shown to significantly reduce individuals’ sensitivity to faces, to an extent likely to impact recognition of familiar faces and facial expressions.29
Several quality of life studies examining AMD have included measures of anxiety and depression. Individuals with foveal vision loss due to AMD are more likely to suffer from depression and report poorer quality of life than individuals without AMD.30–35 Elderly individuals with AMD scored significantly worse on their quality of life, emotional distress35 and depression, with prevalence levels as high as 33%,31,33 especially when the loss of vision was relatively recent35 or there was a perceived lack of social support.36 This depression seems to be related to loss of social function: depressed individuals with AMD reported poorer social functioning as compared to non-depressed individuals with AMD.31
The association between depression and AMD may be stronger than the relationship with anxiety. One study has demonstrated an association of AMD with both depression and anxiety34; however, in a second study, depression but not anxiety scores were found to be strongly associated with visual acuity loss severity.30 In a later meta-study, prevalence estimates from nine cross-sectional and cohort studies found that depressive symptoms were more common than anxiety symptoms in individuals with AMD.37
GlaucomaEarly symptoms of glaucoma (a disease that damages the optic nerve) include a loss of peripheral vision, but in more severe cases of the disease a large proportion of the visual field can be impacted. Once again, quality of life studies that have specifically examined scores associated with psychosocial function have found that glaucoma negatively affect psychosocial functioning, with a higher prevalence of generalized anxiety and depression,38–40 especially in those that lack social support.41
Consistent with the idea that difficulties in face recognition may contribute to depression, the psychosocial impacts of glaucoma are correlated with the extent of vision loss; With increasing glaucoma severity, at levels where face recognition is likely to be impaired, quality of life decreases and depression is more common.42 When comparing progressed glaucoma with severe visual field defects to glaucoma patients in general, patients with severe visual field deficits had a higher prevalence of both depression and anxiety,43 with a linear increase in anxiety as a function of worsening acuity.38
Retinitis pigmentosa (RP)In the earlier stages of Retinitis Pigmentosa (a progressive degenerative disease of the eye characterized by loss of photoreceptors that starts in the periphery of the visual field) symptoms include trouble seeing at night and decreased peripheral vision. As peripheral vision worsens, people may experience "tunnel vision". Once again, multiple studies show that RP is a strong risk factor for depression and anxiety.44–50 Prevalence estimates suggest that approximately 37% of RP patients suffer from anxiety and 15–26% suffer from depressive symptoms.46,49
Once again a correlation is seen between quality of life scores, including social life, anxiety and depression, and the degree of visual impairment, as measured by visual acuity and the residual visual field.46,47,49
Given that individuals with RP retain the ability to recognize faces until very late stages of the disease, difficulties in face recognition are unlikely to be causally responsible for psychosocial distress. Indeed, the causal link may work in the other direction. Worry about disease progression or restricted mobility may be the primary causes of depression – which may in turn reduce social activity. Consistent with this proposed reversed directional relationship, objective visual function does not predict depression scores in individuals with RP (unlike MD), but depressed RP individuals have significantly worse social functioning than non-depressed individuals47, independent of visual loss.
SummaryThus, regardless of the age of disease onset or type of vision loss, visual impairments carry a heavy psychological burden, with a significantly elevated risk of anxiety and depression. This is likely to be driven by multiple factors that include direct visual loss, a fear of worsening vision, loss of employment opportunities, restricted activities and mobility, and difficulties in accessing health care. Even with minimal visual loss, the mere knowledge of having a disease predictive of progressive vision loss can have a negative effect on quality of life.11
However, for most of the diseases described above, correlations are found between the extent of vision loss and psychosocial symptoms, suggesting that the vision loss caused by disease progression worsens psychosocial health. While most studies have not specifically examined social functioning, within at least four diseases (ROP, glaucoma AMD, RP) correlations been found between vision loss, depression and impaired social function.21,28,31,38,47
The link between difficulties in face processing and anxiety and depression - prosopagnosia and autismAnother reason for suspecting that difficulties understanding visual facial information might lead to social anxiety and depression is that analogous phenomena have been observed in the context of prosopagnosia and ASD. Challenges in social interaction due to difficulties processing social information leads to social anxiety, avoidance and depression in individuals with both prosopagnosia and ASD.
The link between prosopagnosia and social anxietyProsopagnosia, also known as face blindness, is an impairment in the ability to recognize faces in the absence of lower-level visual deficits. Individuals with prosopagnosia tend to rely on non-facial visual cues (such as hair or clothing or voice) to identify others.51 Both for acquired and developmental prosopagnosia, impairments in the ability to recognize faces often leads to social anxiety and depression.20,52,53
Individuals with prosopagnosia often feel unable to recognize faces in social settings, leading to significant difficulties in social interactions along with feelings of anxiety about offending others.20 One study using the Cambridge Face Memory Test (CFMT) suggests that difficulties with face recognition abilities specifically results in social rather than general anxiety.16
Prosopagnosia causes long-lasting psychosocial consequences, including changes to behavior to avoid situations in which recognition failure can occur. These can include avoiding social situations, relying on social support to assist in face recognition, stress, social anxiety, personality changes, changes in social relationships and networks, lack of confidence and isolation, and trouble with careers.20 Children with prosopagnosia frequently encounter psychosocial challenges in their lives, including feelings of embarrassment, anxiety, depression, and developing atypical social skills.51,53
The link between ASD and social anxietyASD (a developmental disorder that impairs social behavior) results in subtle specific visual difficulties. ASD leads to altered activity within the cortical temporal face recognition area (fusiform gyrus) and the amygdala - a subcortical area known to be involved in the processing of visual emotional information. Altered connectivity among within a wide distribution of cortical areas associated with face processing has also been observed.54,55 One study found that 36% of individuals with ASD also had prosopagnosia, further worsening social function and potentially increasing social anxiety.56
Unlike individuals with prosopagnosia, individuals with ASD have difficulty with a broad range of social cues. ASD individuals typically also have difficulties in understanding vocal social cues, such as responding to their name being called.17,57 Individuals with ASD also often have difficulties assessing and interpreting language exchange, facial and bodily gestures, posture and body movement.18
These general difficulties in processing social interactions result in social anxiety58, and depression; in a recent meta-analysis of adults with ASD, prevalence estimates of social anxiety were 29%, which included comorbid depression in 23% of individuals.59 This relationship is thought to be bidirectional: The social anxiety of individuals with ASD may cause them to avoid social situations,57,60 thereby further contributing to their social difficulties and depression.61
The potential of auditory rehabilitation trainingTraining in use of auditory cues is now standard practice for individuals who are blind or have very poor vision. Currently this training focuses primarily on mobility and on screen reading; helping blind individuals orient themselves in the world or navigate a computer using auditory cues.62,63
Blind and low-vision individuals generally have faster listening rates than sighted individuals,64–67 and training in using fast listening rates with screen readers (for example JAWS, VoiceOver) is now becoming commonplace.
For people with typical auditory and visual capacities, voice recognition is far worse than face recognition68,69 – face stimuli must be significantly blurred before visual performance is matched to that of auditory performance.70–75 Furthermore, voice recognition is worse after presenting voice distractor items, whereas the ability to recognize faces is robust to the inclusion of visual face distractors.76
Voice recognition relies on a network of brain areas thought to include the caudate and the inferior frontal gyrus (IFG) within the left hemisphere, and the posterior superior temporal gyrus (pSTG), inferior/middle frontal gyrus (IFG/MFG), and the medial frontal gyrus in the right hemisphere.77,78
These voice selective areas are separate from the visual face-processing network, but these two networks show rapid and powerful interactions79–81 during multimodal person recognition.82 For example, interactions between face and voice processing areas occur within 200 ms, response times are shorter for congruent face-voice pairs than for incongruent ones,83 and recognizing familiar voices strongly activates fusiform face regions in sighted listeners.84 In the case of auditory voice training in sighted individuals, auditory voices that were paired with faces during training were recognized with more accuracy. Pairing faces with voices during training also produced an increased functional coupling between face and voice areas.85
Fortunately, in individuals who are habituated to a loss of visual face information, voice recognition does not seem to be impaired by the absence of face information. For example, most individuals with acquired prosopagnosia do not show voice recognition deficits.86,87 Nor does a lack of visual information developmentally impair voice recognition – voice recognition in individuals with developmental prosopagnosia does not differ from controls.88
Moreover, individuals who become profoundly blind or suffer from low vision prelingually are better at voice processing than sighted individuals across a wide range of tasks: They show better voice recognition,89,90 learn new voices faster, and their reaction time in a voice discrimination task is shorter.91,92
One interesting possibility is that enhanced voice recognition as a result of visual loss may be mediated by the recruitment of cortical visual face recognition areas.93,94 Activity within regions of the STS and regions of the fusiform cortex, areas associated with visual face recognition in normally sighted individuals, show task-dependent activity to voices in both early and late blind but not sighted subjects.79,91,92,95–97
As far as rehabilitation training is concerned, it seems plausible that the ability to decode social cues like identity and emotion from voice alone could be improved further with training. Indeed, experts trained in forensic speaker identification, are better at voice identification than non-experts.98,99
The analogy with speech-reading for hearing lossWhen deafness results in a deterioration in the ability to communicate there are substantial psychosocial consequences. As a result, training in speech-reading (training in the effective use of visual clues of the speaker's lip and facial movements, gestures, posture and body language100, has become increasingly popular as a rehabilitation strategy for hearing loss,101–104 for review see.105,106 Visual speech-reading training improves speech recognition,107 in both early deaf individuals100 and individuals who have lost hearing later in life.108,109 Furthermore, speech-reading, by improving communication skills, improves aspects of psychosocial functioning.108,110 It seems plausible that an analogous approach, training blind individuals in voice recognition, might lessen social difficulties and anxiety due to blindness.
ConclusionAs described above, it is now well-established that low vision and blindness is a risk factor for anxiety and depression. This relationship has been attributed to multiple factors, including anxiety about disease progression, and a lack of access to work and social activities. However, we believe a potential additional contributing factor, that has been hitherto overlooked, is that blindness may make it more difficult to effectively engage in social interactions, due to a loss of visual information about facial identity and expression.
As described above, several studies in the literature suggest an association between blindness and impairments in social function.21,28,31,38,47 However a critical gap in the literature is that all of these studies have tended to focus on specific blind populations, have used a wide variety of instruments to measure visual impairment,111 and have used a wide variety of quality of life instruments, that vary in their measurement of social function, social anxiety and depression. This heterogeneity precludes collating quantitative data across studies to infer the effects of different types of vision loss on social anxiety and depression. Previous meta-reviews have similarly noted the need for a unified definition for quality of life, and studies better targeted towards understanding the specific associations between particular types vision impairment and varied measures of well-being.9
Generally, rehabilitation for low vision and blindness has focused on112 improving reading speeds,113 training in the use of assistive technologies such as magnifiers, orientation and mobility skills,114 and developing assistive computer skills (e.g. JAWS).115,116 In this review, we suggest that the additional inclusion of rehabilitative training in voice recognition might lessen social difficulties and anxiety due to blindness.
It has already been demonstrated that early blind individuals have enhanced voice recognition compared to sighted individuals. In the case of early blind individuals this enhanced voice recognition may be the result of developmental cross-modal plasticity. However, work in adult sighted individuals shows that voice discrimination can improve with training, even in adulthood. Rehabilitation training in voice recognition and processing vocal social cues is therefore likely to be effective in late blind individuals, even if these improvements have a different neural basis from the enhanced performance observed in early blind individuals.
Finally, we believe an important future direction will be developing better models of how blindness impacts mental health. To date, most studies of depression, anxiety, emotional distress and social anxiety in early blind individuals have had an ophthalmological perspective. As a result, these studies have tended to focus on assessing the prevalence of psychosocial symptoms via relatively broad psychiatric subscales. This approach leaves the causal relationships between vision loss and psychosocial impairments a mystery, making it difficult to effectively target psychosocial treatment. Individuals with AMD and RP may both suffer from similar levels of depression and social isolation – but the causal pathways may be very different.
A closer examination of how different kinds of vision loss impair different types of activities and have varied psychosocial consequences has the potential to provide important new insights. With richer and more detailed data sets, it would be possible to use more sophisticated models, such as dynamic latent variable analyses,1 network analyses, and structural equation models, to generate a better understanding of comorbidities, risk factors, and potential mediators of the psychosocial distress caused by blindness. More informed models could be used to better guide both rehabilitative intervention and the clinical treatment of psychosocial distress.
NIH National Eye Institute Grant R01EY014645 (to I.F.) and an Envision Fellowship award (to S.K.)