Signals from the peripheral retina are important for myopia development. Unlike temporal vision, deficits in peripheral spatial visual functions of myopes have been investigated previously. This study investigated temporal contrast thresholds in emmetropes and myopes at different retinal eccentricities.
MethodsForty-four young adults (mean age 23 ± 3 years) including 21 emmetropes (Spherical Equivalent (SE): +0.01 ± 0.30D) and 23 myopes (SE: -3.98 ± 2.41D) participated in this prospective study. Flicker modulation thresholds (FMT) were determined monocularly (right eye) for 15 Hz flicker stimulus at 0°, nasal (23°, 10°) and temporal (-23°, -10°) retinal eccentricities along the horizontal meridian. FMTs were measured psychophysically using 5-adaptive interleaved staircases and threshold was taken as the average of the last 6 reversals.
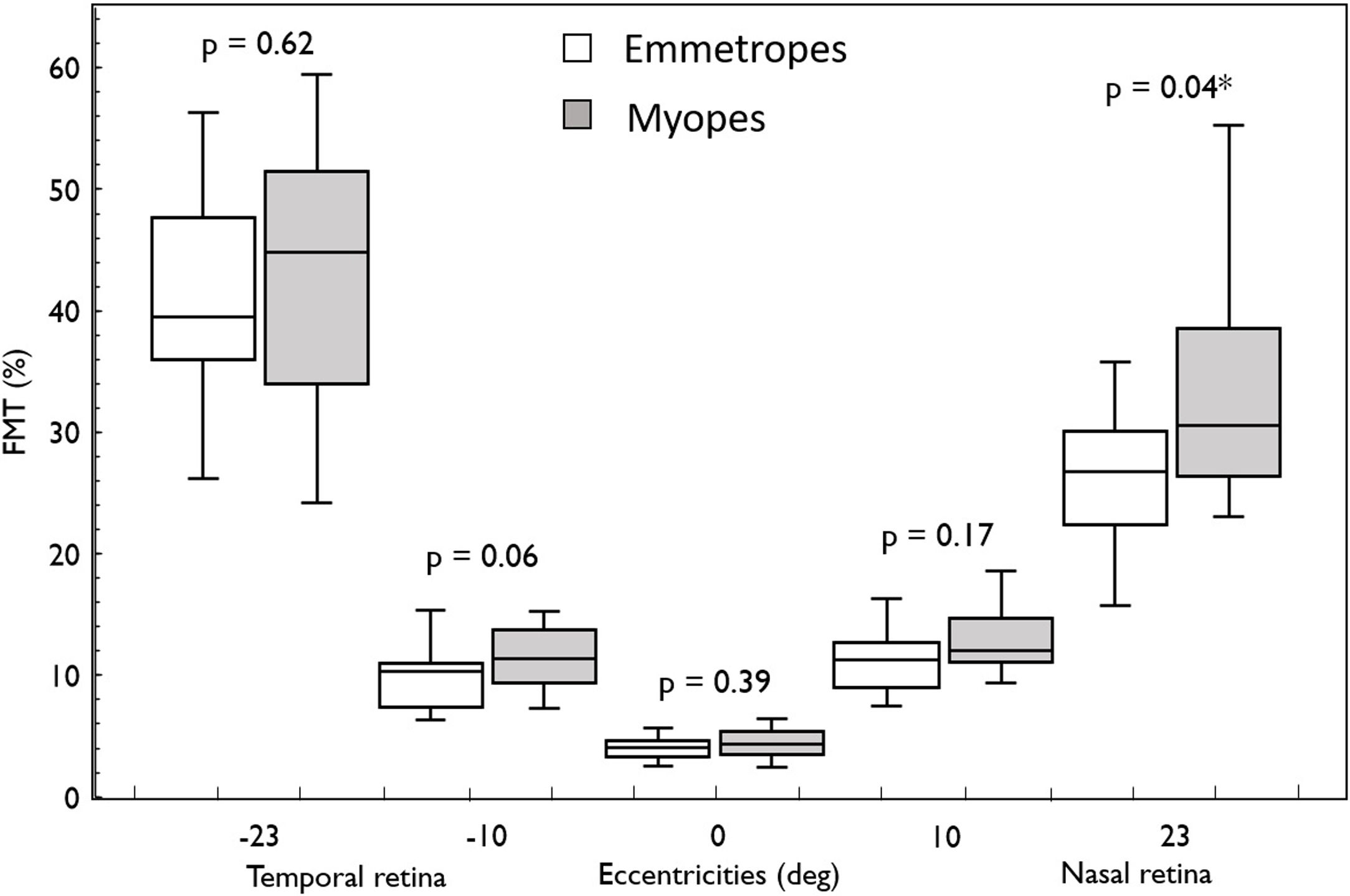
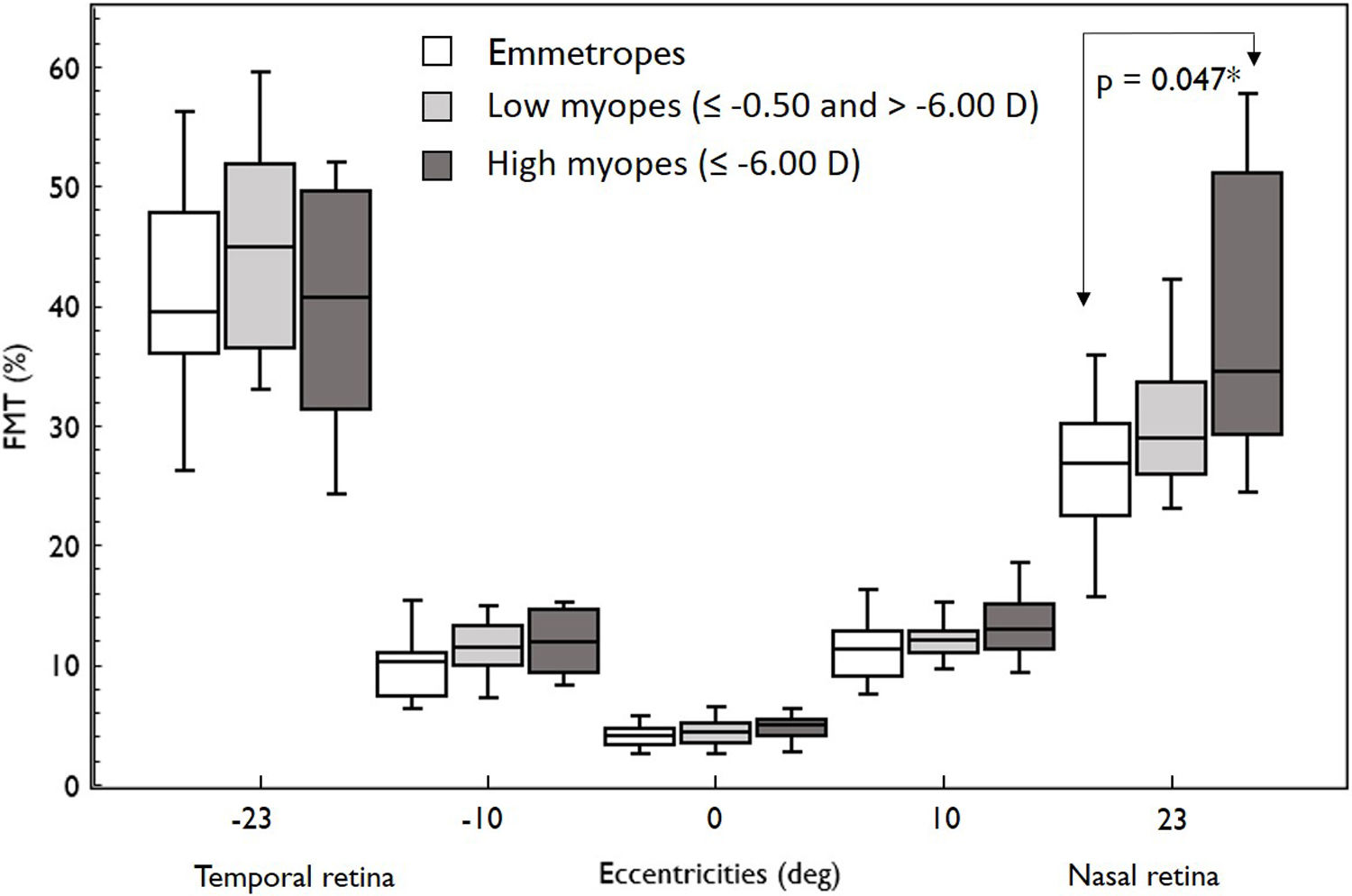
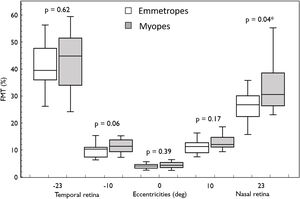
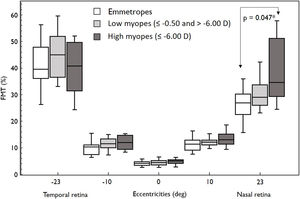
ResultsIn both the groups (emmetropes and myopes), there was a naso-temporal asymmetry in FMTs with higher thresholds in the far temporal retina (Median; Interquartile range: 40.97%; 17.06) than the nasal retina (28.07%; 9.36) (p < 0.001). Flicker modulation thresholds were significantly higher in myopes (30.58%; 12.15) compared to emmetropes (26.77%; 7.74; p = 0.04) at far nasal retina (23°), while at other eccentricities there was no effect (p > 0.05). Further sub-analysis revealed only high myopes (34.48 %, 21.9) showed significantly higher FMT compared to emmetropes (26.77%; 7.74; p = 0.04).
ConclusionGreater FMTs were seen in high myopes than that of emmetropes in the nasal retina. Further studies exploring the structural aspects of the myopic eye with FMT would provide a better understanding of role of flicker sensitivity in myopiogenesis.
Myopes are known to exhibit deficits in visual functions including visual acuity,1 blur detection2 and contrast sensitivity (spatial and temporal)3,4 at both centre (fovea) and more prominently in the periphery of the eye.1,5 Given the potential role of the peripheral retina in myopia development,6 there is more focus on understanding the various visual functions in myopes in the peripheral retina. Peripheral visual acuity was found to decrease drastically in myopes, which has been attributed to the peripheral retinal stretching.1 Similarly, defocus (1D) reduces contrast-detection acuity by ∼50% in the peripheral retina (20°).2 Spatial contrast thresholds in myopes at different retinal eccentricities (± 30°) were reported to be significantly higher than that of emmetropes.5
Compared to the spatial domain, literature is sparse with regards to temporal contrast sensitivity in myopes. There are previous reports on the comparison of central temporal contrast sensitivity between myopes and emmetropes. However, it remains ambiguous. While Chen et al.4 reported higher foveal temporal flicker thresholds in high myopes (> 8 D) than that of low myopes (< 2 D), Comerford and colleagues7,8 did not find any significant difference between emmetropes and high myopes. None of the previous studies has studied flicker modulation thresholds in myopes in the peripheral retina. However, objective techniques such as peripheral retina mfERG (multifocal electroretinography) responses showed delayed implicit times in high myopes than that of emmetropes suggesting temporal deficits in myopes relative to emmetropes.9 Besides, flicker is a sensitive stimulus to detect retinal changes before evident structural damage in various retinal diseases10 and therefore it may be useful to investigate the association between peripheral temporal contrast thresholds and myopia. This study investigated how flicker thresholds (15 Hz) vary in individuals with emmetropia and varying degrees of myopia at different horizontal retinal eccentricities (0°, ±10°, ± 23°). Based on the previous psychophysical11 and electrophysiological findings,9 we hypothesize that there will be deficits in flicker sensitivity in myopes compared to emmetropes in the peripheral retina.
MethodsThis is a prospective experimental study. The study protocol and ethics for the study were approved by the Institutional Review Board of L V Prasad Eye Institute (Ref: LEC 04-18-048) (LVPEI), India and it adhered to tenets of the Declaration of Helsinki. Written consent was obtained from all participants after explaining the nature of the tasks involved in the study. Participants were the staff and students of LVPEI and were recruited based on the following inclusion criteria: Age ≥ 18 years, spherical refractive error between +0.75 D to -14.00 D with cylindrical power ≤ 1.50 D as observed in final subjective refraction and best-corrected visual acuity 20/20 or better. Any participants who had any ocular or systemic conditions that could influence the refractive error were excluded from the study. Participants were classified as emmetropes (spherical equivalent (SE) between +0.75D to > -0.50D) and myopes (SE ≤ -0.50). Based on the International Myopia Institute (IMI) guidelines12 myopes were further classified as low myopes -0.50 D to -5.75 D and high myopes ≤ -6.00 D. The refractive error criteria was applicable for both the eyes, however only right eye was considered for measurement. Axial length data was available in a subset of 18 participants (8 emmetropes and 10 myopes) from this study who had also participated in a larger study in the myopia lab.
Flicker-plus testMonocular Flicker Modulation Thresholds (FMT) were measured centrally and at eccentricities (nasal retina 10° & 23°, temporal retina -10° & -23°) in the horizontal meridian using a custom-built approach in the Flicker-plus module13 of the Advanced Vision and Optometric Tests (AVOT) (City Occupational, UK). The setup consists of a laptop and a display monitor that is separated by a black curtain to prevent any stray light entering the eye. The Flicker-plus module in the laptop was operated by the examiner to present the stimuli in the display monitor for the participant. The stimulus display monitor was a spectrally calibrated (EIZO, Model ColorEdge CS2420; EIZO Corporation, Japan) using a photometer. The display resolution was 1600 × 1200 and a frame rate of 120 Hz.13
The background and target chromaticity comprised of long-wavelength light (CIE 1931, x = 0.58, y = 0.36) to minimize the absorption of short-wavelength by the crystalline lens and the macular pigment.13 Background luminance was 32 cd/m2 and the starting contrast was 20%. The stimulus is a uniform flickering disc of size 25′ (arc minutes) subtended at a distance of 66 cm and the stimulus was presented for the duration of 334 ms. The temporal frequency of 15 Hz was chosen considering that this frequency is most sensitive to detect any early retinal functions defects.13
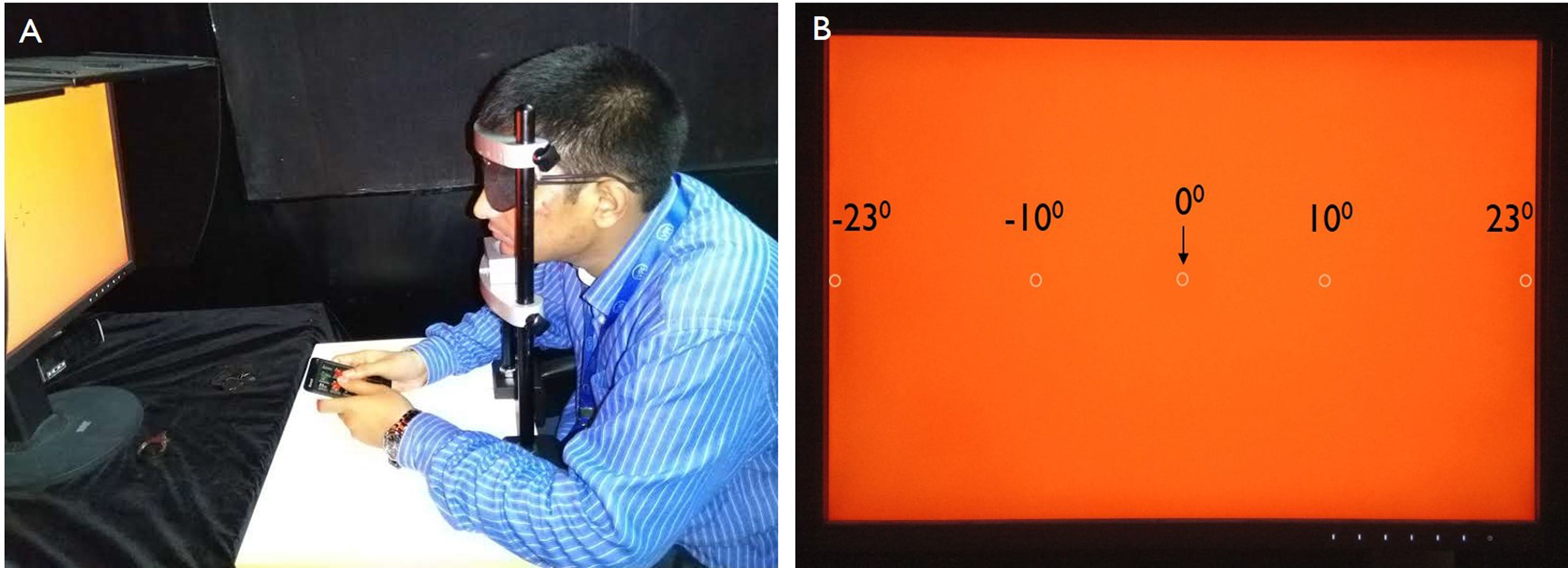
Monocular (right eye) measurements were obtained from the participants, who seated at a distance of 66 cm from the display monitor with their head resting on a custom-built head-chin-rest (Fig. 1A). The non-tested eye (left eye) was occluded for viewing using an eye patch. Each test run measured FMT in five locations, one in central vision (0°), two points in the nasal retina (23°, 10°), and two points in the temporal retina (-10°, -23°) as shown in Fig. 1B. The farthest eccentricity (23°) that could be tested was limited by the width of the monitor and the testing distance. We did not test 15° stimulus in the temporal field to avoid possibly stimulating the blind spot and for consistency and ease of comparison, testing at 15° nasal was also not performed. The stimulus was presented randomly in any of the five stimulus locations and the FMTs at each location were measured using a 5-Alternate-Forced-Choice test.13 A separate numeric keypad was utilized to the participants to indicate the location of the stimulus in the monitor. The participants were instructed to focus on the central blinking square to maintain fixation throughout the test. The threshold was determined using a 2-down, 1-up procedure, and FMT of each run by the average of the last six reversals.13,14 Two such runs were averaged to obtain the final threshold for each participant. Each run required approximately 7−8 min for completion.
Experimental set up for measuring flicker thresholds in emmetropes and myopes at different retinal eccentricities. (A) The subject is performing the test to identify the location of the stimulus at different retinal eccentricities to measure the flicker thresholds with the help of keypad. (B) The appearance of the screen view when the test is on and the locations of the stimulus present at different retinal eccentricities. The circle in the panel B indicates stimulus locations.
Statistical analyses were calculated in IBM SPSS Statistics Version 21 (IBM SPSS Statistics, Armonk, NY) and the figures were created with in-built features of Microsoft- Excel 2016 (Microsoft Corporation, Albuquerque, New Mexico, United States). The Shapiro-Wilk test for normality indicated that the data was not normally distributed (p < 0.05) and therefore, non-parametric tests were applied to check the statistical significance. Variability between sessions was assessed using the Coefficient of Variation (COV). Mann-Whitney U test was performed to compare the data between the independent groups. Friedman’s test was performed for FMT comparison different between each of the retinal eccentricities within each refractive error groups. Post-hoc test was applied for pairwise comparisons. Spearman correlation coefficient was used to determine the relationship between mean refractive error and the FMT at all the retinal eccentricities. The criterion for the statistical significance was set as p < 0.05.
ResultsA total of 44 individuals (8 males & 36 females) with mean ± standard deviation (SD) age of 23.41 ± 3.45 years participated in this study. There were 21 emmetropes (+0.01 ± 0.30 D) and 23 myopes (-3.98 ± 2.41 D), which included low myopes (n = 17, SE: -2.90 ± 1.70 D), and high myopes (n = 6, SE: -7.06 ± 0.91 D).
In this study, ∼ 93 % (205/220) of the test conditions (N = 44, number of eccentricities = 5) showed ≤ 20% of the Coefficient of Variation (COV) between the two sessions for the same participants. There were significant differences in flicker modulation thresholds between central and peripheral locations (near and far) (Friedman test; χ2 (4) = 168.52, p < 0.001). FMTs were significantly higher in both the far peripheral retinal (23°) eccentricities ((Median) at nasal: 28.07 % and at temporal: 40.97 %) compared to that of central retina (0°; 4.18%; p < 0.001). The FMTs at near peripheral retinal (10°) eccentricities ((Median) at nasal: 11.98 % and temporal: 10.72%) were also significantly higher than central retina (4.18%; p < 0.001). Overall, the FMTs were significantly higher in the temporal retina than that of the nasal retina (40.97% vs. 28.07%) and were statistically significant (p < 0.05).
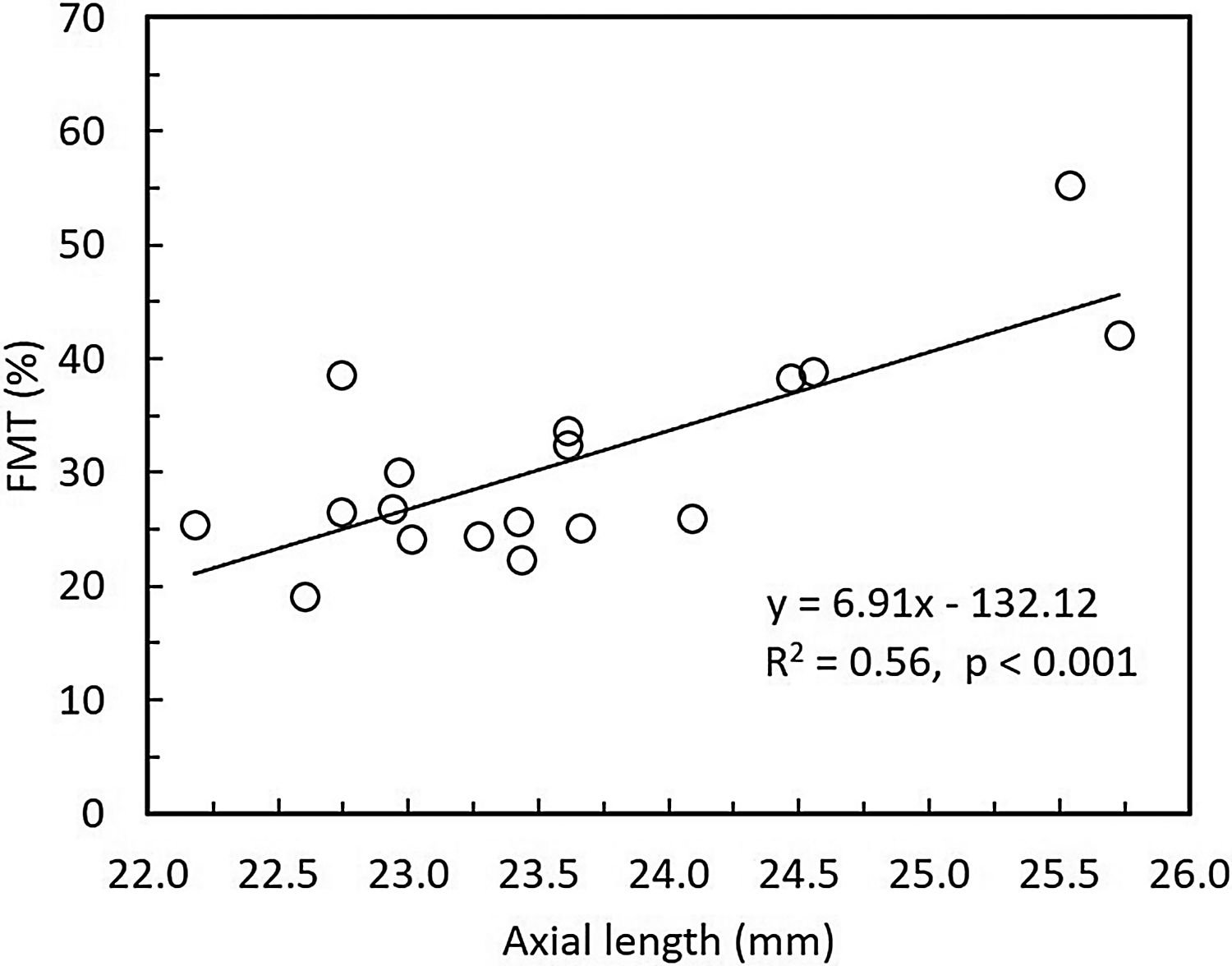
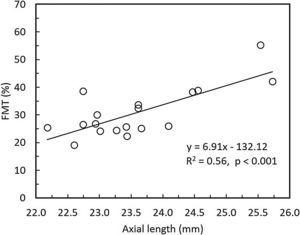
Overall, FMT in myopes (30.58%) was significantly higher than that of emmetropes ((26.77); p = 0.04) only at nasal retinal 23° eccentricity (Fig. 2). On further post-hoc analysis, investigating differences in sub-groups (emmetropes, low and high myopes), Mann-Whitney-U test showed a significant difference in FMTs between emmetropes and high myopes (p = 0.047) (Fig. 3) but not with other subgroups (p > 0.05). Nasal location FMTs (23° and 10°) showed significant relationship with axial length (R2 = 0.56, p < 0.001 (Fig. 4) and R2 = 0.29, p = 0.02 respectively). However central and temporal FMTs (23° and 10°) did not show significant relationship with axial length (p > 0.05). Spearman correlation between the central or peripheral flicker thresholds and refractive error (SE) was not statistically different (central ρ = -0.02, p = 0.93; nasal 23°: ρ = -0.02, p = 0.94; temporal 23°: ρ = 0.16, p = 0.47).
Box and Whisker plots showing median flicker thresholds in emmetropes and myopes at different retinal eccentricities on the horizontal meridian. The graph indicates higher flicker thresholds from central (fovea) to the peripheral retinal eccentricity and also showing higher flicker thresholds in myopes compared with that of emmetropes in all retinal eccentricities. Nasal retina (23°) FMTs were significantly higher in myopes compared with that of emmetropes (p < 0.05, indicated by asterisks*).
Box and Whisker plots showing median flicker thresholds in emmetropes, low myopes, and high myopes at different retinal eccentricities on the horizontal meridian. The figure indicates higher flicker thresholds in high myopes at all retinal eccentricities compared to emmetropes. Nasal retina FMTs were significantly higher in high myopes than that of emmetropes (p < 0.05, indicated by asterisks*).
This study revealed three main findings. Firstly, FMT increased significantly in the far retinal periphery compared to that of the central location. Secondly, there was a naso-temporal asymmetry in FMTs in both myopes and emmetropes. Finally, the most important finding from the study is that FMTs at the far nasal retina (23°) were significantly greater in high myopes than emmetropes.
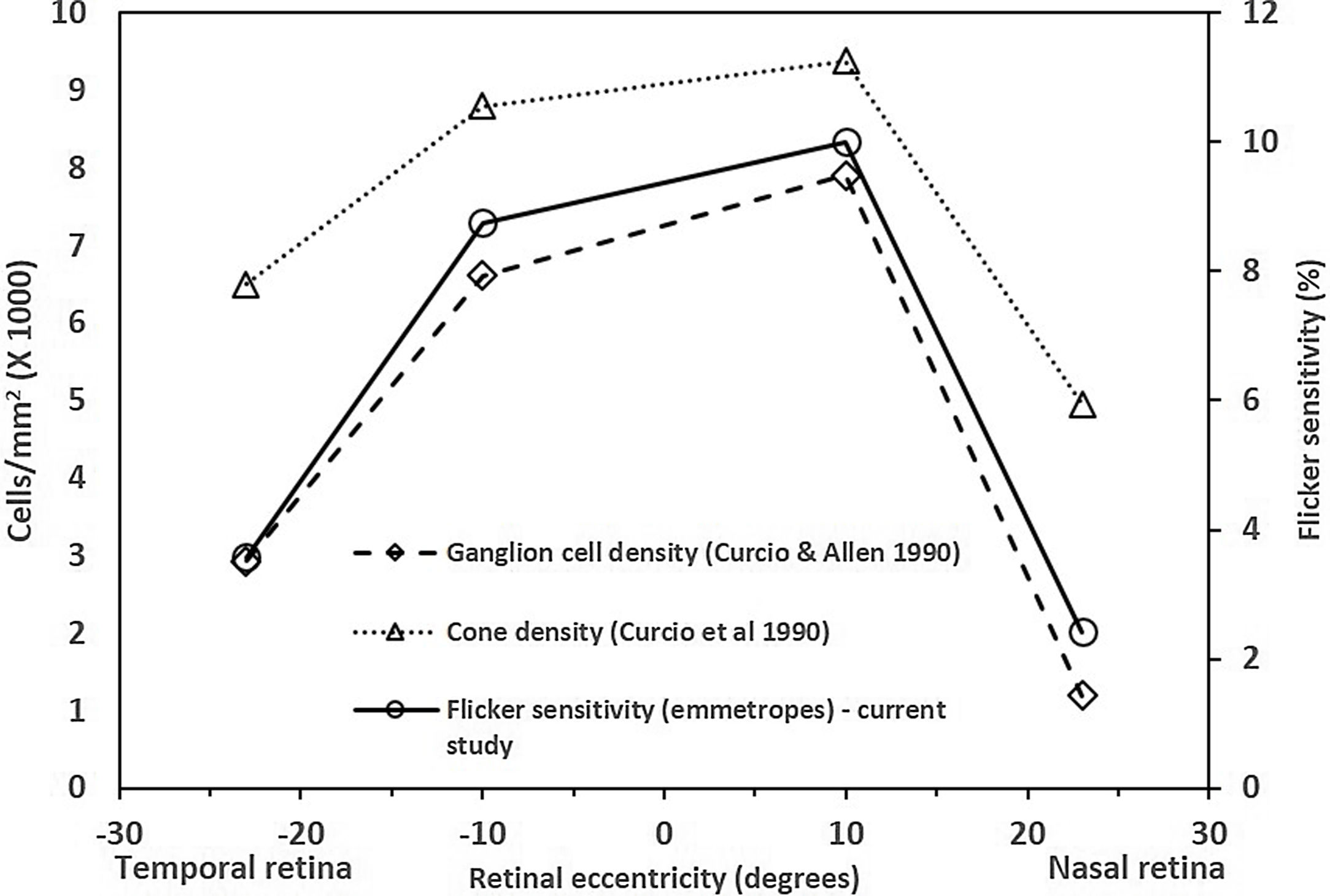
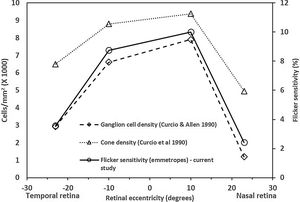
The central FMTs are consistent with the previous age-matched normative database13 obtained using the same experimental setup. The FMTs were significantly higher at the far periphery compared to that of the central location, including the asymmetry in naso-temporal FMTs. These findings are consistent with previous studies related to flicker thresholds and retinal eccentricity.10,15 The naso-temporal asymmetry in FMT agrees well with findings of Grigsby et al.,15 who reported significantly higher FMT at far eccentricities (24° & 32°) in the temporal retina than in the nasal retina. Previous studies have indicated an asymmetrical decrease in cone density16 and ganglion cell density17 in the naso-temporal retina. Therefore, to identify the possible retinal substrate, we plotted the average flicker sensitivities (1/ FMT) of the emmetropes from this study, cone and ganglion cell densities of a young cohort obtained from the literature17,18 as a function of retinal eccentricities (shown in Fig. 5). Based on the results, it appears that flicker sensitivity may be driven more by retinal ganglion cells than the cone photoreceptors. Besides, the consistencies of FMT changes with increases in eccentricity with previous literature could be considered as a measure of validation of the FMT measurements obtained using the current set up.
The findings from this study that similar FMTs between emmetropes and myopes at the central retina are in agreement with that of two other studies7,8 but not with the findings of Chen et al. (2000)4 carried out in East Asia, who had included only high myopes. It is possible that the potential differences in the retinal shape among ethnicities19 could have attributed to the differences in functional changes in temporal contrast sensitivity. Intriguingly, no previous studies have examined how peripheral flicker thresholds will vary between emmetropes and myopes, and to the best of our knowledge, this study is the first to explore the same. Therefore, direct comparisons of the findings from this study cannot be made with the existing literature. We found significantly higher FMT in high myopes than in emmetropes only at nasal retinal 23° eccentricity. This reduction in flicker sensitivity could be discussed in relation to optical, structural or neural/retinal substrates. Firstly, the relative peripheral refractive error is known to vary with refractive error type (high myopes show relative peripheral hyperopic defocus and emmetropes show relative peripheral emmetropia or mild myopia) will degrade the peripheral image quality1 and thus visual performance in the peripheral retina.20 Besides, studies have also reported naso-temporal asymmetry in both peripheral refraction and retinal shape which could have also led to the differences in the FMTs between nasal and temporal retina.19
Secondly, the role of the retina in myopiogenesis and implications have been studied using electroretinographic (ERG) techniques including full-field electroretinography, pattern ERG, and multifocal ERG.9,21 The multifocal ERG implicit times in peripheral rings (9.3–19.8°) are delayed in myopes than emmetropes9,21 and reduced amplitudes,9 which could indicate towards inner retina (ganglion cell dysfunction). However, there are no reports of ganglion cell loss as a function of eccentricity in myopes compared with that of emmetropes. There is only evidence of a decrease in ganglion cell density in high myopes compared to emmetropes, in the peripapillary region,22 which however cannot implicate the current study findings. However, it has been previously reported that myopes may have deficits in the magnocellular pathway,11,23 which is primarily present in the retina periphery24 and likely to be responsible for processing high temporal frequency (≥ 15 Hz).25 The possible reason for implicating ganglion cells in MC pathway is primarily because they are relatively low in number compared to PC pathway ganglion cells26 and overall loss in ganglion cell loss is likely to impact MC pathway due to larger proportion of the loss, which could lead to a functional loss.11 Finally, another hypothesis for higher FMT in high myopes could be associated with altered morphological retinal circuitry27 or low L/M (long wavelength-sensitive (LWS)-to-middle wavelength-sensitive (MWS)) cone ratio,28 compared with that of emmetropes due to the retinal stretching caused by the posterior pole expansion which may reduce the retinal cell responsivity29 which in turn could affect visual performance in the axial myopic eyes as noted previously.1 The sensitivity would also drop, if the neural limit due to retinal stretching falls below the optical cut-off.30 The FMT did not show a significant association with the magnitude of the refractive error at all retinal eccentricities in the current study. However, nasal FMTs (23° and 10°) showed significant relationship with axial length. The lack of relation between temporal FMTs (-23° and -10°) and axial length might be related to naso-temporal asymmetry in retinal shape in emmetropes and myopes.19 There is only one study that has reported a weak relation between a measure of temporal processing (CFF frequency) as a function of refractive error (r = -0.36, p = 0.04) and axial length (r = 0.33, p = 0.06).31 The lack of correlation could be because temporal processing deficits may occur only in high myopes.
The limitations of the present study are as follows: 1) the absence of an eye tracker to observe the movements of the eye to ensure the fixation; however, the patient was constantly reminded to maintain fixation on the target throughout the test. 2) Peripheral flicker stimulus size was not compensated for cortical magnification. However, if the lack of m-scaling of stimulus size affected FMTs, it would have affected thresholds at all the retinal eccentricities. However, only the far nasal (23°) FMTs are greater in high myopes than that of emmetropes. Therefore, FMT differences noticed are unlikely to have contributed due to lack of size scaling. 3) Peripheral refraction was not measured in the subjects and it would have been useful additional measure to correlate with FMT.
ConclusionIn summary, this study result demonstrates that high myopes exhibited increased flicker contrast thresholds at far nasal retina than that of emmetropes, which may be indicative of potential retinal function deficits that are not yet visible clinically. However, further studies are required in a larger cohort of high-myopes and continual follow-up may potentially help to identify “at-risk” high myopes who are likely to undergo retinal degenerative changes. Future studies also need to explore the relationship between FMT and other myopiogenic factors.
FundingThis research study was financially supported by Hyderabad Eye Research Foundation and DBT CORE Grant (BT/PR32404/MED/30/2136/2019).
Declarations of interestNone.
The authors gratefully acknowledge the Hyderabad Eye Research Foundation - LVPEI for support in conducting this study. We thank the participants from LVPEI for volunteering for their participation in the research study.