In recent past, major pandemics and epidemics have occurred due to the emergence and resurgence of the novel strains of viruses like Influenza [HINI-A “Spanish Flu”, H1N1-Novel A “Swine flu”], Corona [Middle East Respiratory Syndrome (MERS), Severe acute respiratory syndrome (SARS-CoV-1, SARS-CoV-2)] and Ebola virus [Ebola virus disease].
These infectious diseases may have several ocular manifestations and rarely might be the presenting symptom of the underlying disease. The eyes can act as a portal of entry and/or route of viral transmission for these pathogens. Therefore, an ophthalmologist/optometrist needs to act with ample preparedness and responsibility. Establishing a standard of care in ophthalmic practice by modifying the conventional examination techniques and adopting tele-ophthalmology model to triage the patients can control the community spread of the disease. This article aims to elucidate the ocular manifestations in these pandemics and measures that should be adopted in ophthalmic practice to prevent the disease transmission.
Pandemic outbreaks have been known to occur on and off since time immemorial, that have not only handicapped the communities socially and economically but also accounted for heavy death tolls. Ocular manifestations are a common occurrence in most pandemics or epidemics. The reference of ocular involvement dates back to the earliest known pandemic that erupted in Athens in 430 BC, popularly known as the ‘Plague of Thucydides’ who described it as “Violent heats in the head;redness and inflammation of the eyes; throat and tongue quickly suffused with blood;..”
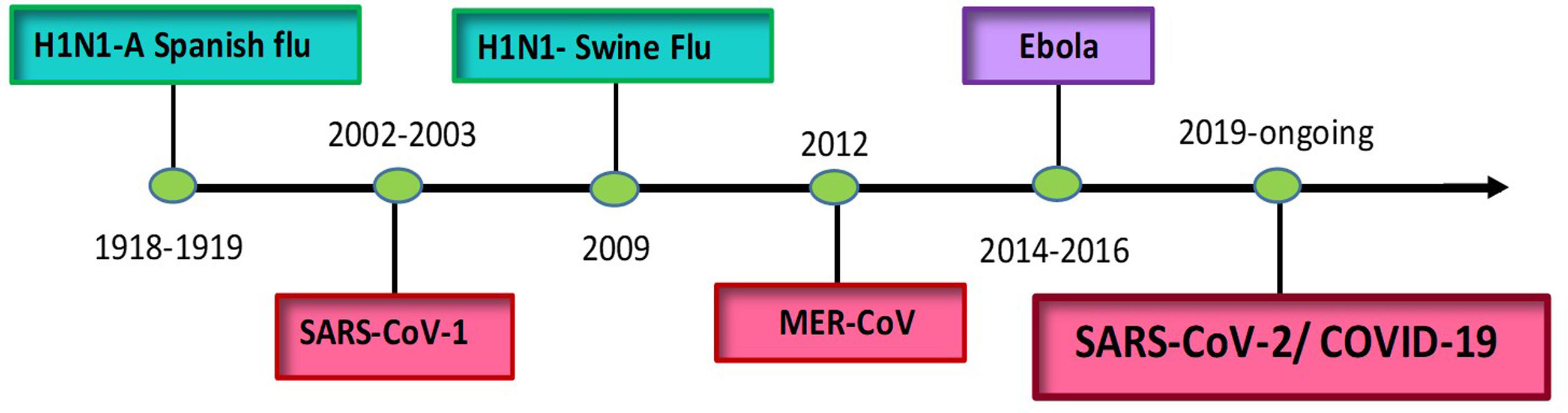
In the last century, the major culprits have been Influenza, Ebola and Corona viruses (Figs. 1 & 2). Their systemic features have been well dealt with in several articles. In this review, we will limit our discussion to the ocular manifestations of recent infectious pandemics and measures that should be adopted in ophthalmic practice to prevent the disease transmission. The search of published literature for this review article was done using Pubmed, Medline, Embase, and Ovid extending over the period of several pandemics and later, along with checking for various cross references. As many articles were not available in the above-mentioned searches, we also accessed the grey literature and other indexing systems like Index Copernicus to make the search more inclusive. English language articles with full text access were preferably included and electronic literature search was performed using key words like viral pandemic, ocular features, influenza, Ebola, coronavirus, prevention and management.
Exploring the pandemicsInfluenzaThe influenza virus is one of the most common strain known to cause epidemics and occasionally pandemics. The route of infection is mainly via the respiratory tract that contains the viral receptors α2,6 sialic acid. The primary manifestations range from mild flu-like disease (fever, malaise, cough, sore throat) to severe life-threatening complications such as acute respiratory distress and multi-system failure. The disease severity is greater in elderly, immunocompromised individuals and pregnant females. Interestingly, ocular surface exhibits tropism for some avian strains of the virus due to the presence of receptors α2,3 and α2,6 linked sialic acid 1
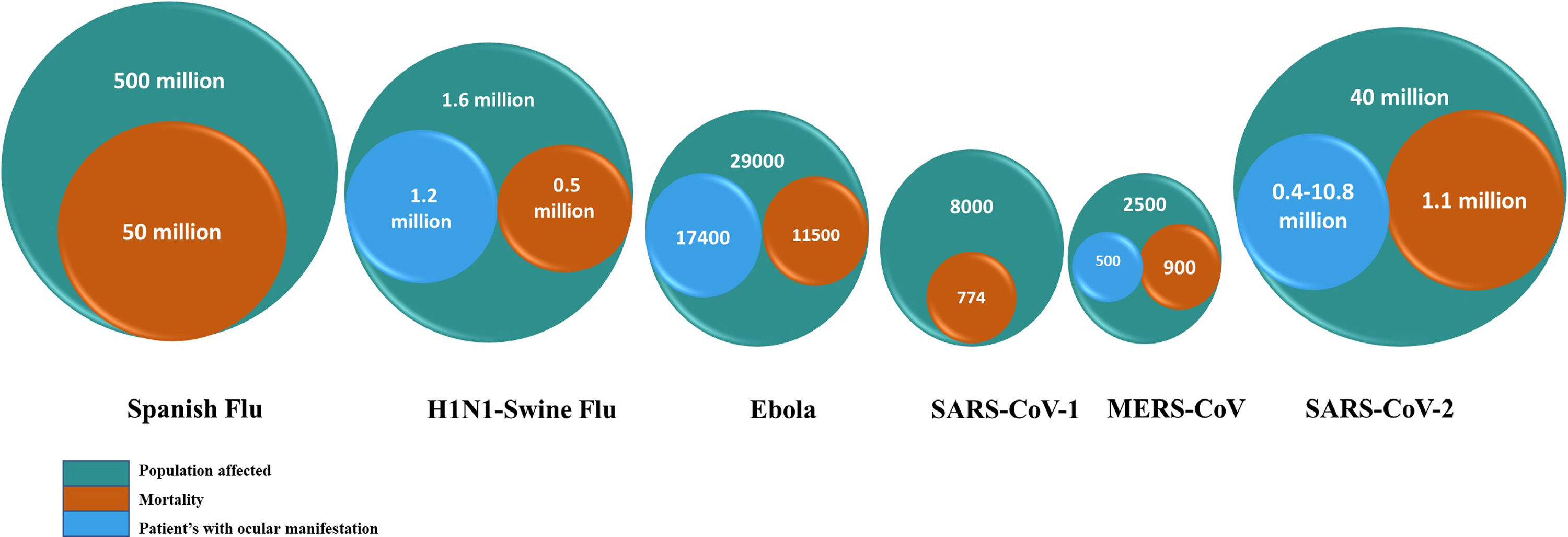
HINI-A “Spanish Flu”This was one of the grave pandemics in human history that started in 1918 and accounted for nearly 50-100 million deaths worldwide over a span of 2 years. The disease spread was facilitated by the troop movements during World War I and young adults were the most affected.
In the early 20th century, isolation of this virus revealed H1N1-subtype of influenza-A strain that had the typical hemagglutinin (HA) and neuraminidase (NA) surface proteins responsible for facilitating entry into the host cells 2,3
Although there is no evidence of direct involvement of the eyes, there are subtle references like “hemorrhages occurring in different parts of the “interior of the eye” 4 and “cases of subconjunctival hemorrhage”.5 This may have resulted from extensive internal and external bleeding that was a likely complication of the disease. Other ocular features like intense pain in the orbit, limitation of ocular motility, accommodation deficits and paralysis of ciliary muscle were mostly seen in relation to the neurological complications of influenza 6
H1N1-Novel A “Swine flu”Next major outbreak caused by H1N1 virus was seen in 2009. Beginning in Mexico, it spread first in the United States and later worldwide infecting nearly 1/3rd of the world’s population with a death toll of around 1.5 to 7 lakhs in the first year. Nearly 80% of the deaths were seen in the young, as the virus was relatively new to this cohort who lacked immunity to this strain unlike the older population 7
This Novel A/H1N1 virus likely evolved by the process of genetic reassortment of multiple strains of swine influenza viruses and hence the name “swine flu”. Studies have shown that this particular strain of H1N1 had the ability to replicate in the human conjunctiva, unlike the seasonal strain, making ocular surface an important route of infection 8 The virus was also isolated from other ocular tissues like cornea, trabecular meshwork and RPE cells. 8–10
Therefore, myriads of ocular features were observed during this pandemic. As per the study from Egypt, nearly 65% cases with novel A/H1N1 influenza developed bilateral acute conjunctivitis with extensive lid swelling and follicular reaction. Other features observed were retinopathy in 4.5%, uveal effusion syndrome in 8%, and optic neuritis in 3.4% cases. The affected eyes of these patients had a positive conjunctival smear for the virus on reverse transcriptase-polymerase chain reaction (RT-PCR) and cytological features were suggestive of viral infection on impression cytology 11 Other less commonly reported features were keratoconjunctivitis sicca, 12 bilateral corneal erosions 13 and granulomatous iridocyclitis. 14 Posterior segment involvement was seen in the form of disc hemorrhages, macular hemorrhages or macular ischemia. 15 Unrelated to the epidemic various other manifestations described with H1N1 strains like bilateral angiopathy, macular edema, frosted branch angiitis, 16 acute multifocal placoid pigment epitheliopathy (APMPPE) and serous macular detachment (SMD) should be kept in mind. 17 Post-vaccination some rare complications like acute disseminated encephalomyelitis (ADEM), bilateral optic neuropathies, 18 and permanent vision loss may occur. 19 The changes could be caused either by the direct cytopathic effect of the virus on ocular tissues or by an immune-complex mechanism.
Apart from emphasizing personal and hand hygiene measures, the Centre for Disease Control and Prevention (CDC) recommends the use of neuraminidase inhibitors like Oseltamivir and Zanamivir for the systemic disease and supportive therapy for ophthalmological manifestations 20
Ebola virus diseaseEbola virus disease first erupted in 1976 as cluster of hemorrhagic fever cases along the Ebola River in Zaire. The largest outbreak was seen in West Africa in 2014 that proved to be highly fatal with a mortality rate greater than 40% 21
Ebola virus is a single-stranded RNA virus of the family Filovirus. Fruit bats are natural reservoirs of the disease. Human to human transmission is known to occur through contact with body fluids or objects contaminated with it. The chance of transmission is even higher in hospital settings, thus specifically placing the health care workers at risk.
The systemic manifestations of the disease are fever, sore throat, abdominal pain, diarrhea, arthralgia, conjunctival congestion and in severe cases bleeding diathesis and shock leading to death.
Conjunctival involvement may be seen in around 60% cases with acute disease and the involvement is usually bilateral. Hemorrhagic conjunctivitis, when present, is predictive of severe disease. Uveitis is mostly seen in the convalescent phase of the disease 22 The virus has been isolated from aqueous humor of a patient who developed scleritis and vitritis after the recovery of the systemic disease and disappearance of the virus from body fluids like blood and urine, raising concerns about the persistence of the virus in the eye. 23
Bilateral conjunctivitis is one of the key features seen in the acute phase, and such patients may often present to an ophthalmologist at first instance. Hence, the ophthalmologists working in an endemic area should be vigilant enough when examining a case of hemorrhagic conjunctivitis 22 As per the CDC recommendations, all health care staff involved in patient care of a confirmed or suspected case of Ebola disease must adhere to standard contact and droplet measures that include hand hygiene and use of personal protective equipment (PPE) to protect the mucosal surfaces from exposure. 24
CoronavirusCoronaviruses are large, enveloped, single-stranded positive-stranded RNA viruses belonging to the Coronaviridae family. They are unique in having the largest genome amongst RNA viruses and the presence of spike protein, the large surface protrusions that give the appearance of a crown. This spike protein is responsible for its entry into the host cells. Numerous strains circulate in various animal reservoirs that can occasionally break the interspecies barrier and infect humans. There are 7 variants known to infect humans and three out of them have led to major pandemics of this century - MERS-CoV causing Middle East Respiratory Syndrome (MERS), SARS-CoV causing SARS, SARS-CoV-2 causing COVID-19. These are all novel strains belonging to the genus Betacoronavirus with unknown animal reservoirs. The systemic features range from mild seasonal flu-like symptoms often culminating to severe pneumonia and respiratory distress due to lung involvement in the form of bilateral multifocal air-space consolidation or infiltration. There are also reports of multi-organ failure and kidney dysfunction. Greater disease severity is observed in the elderly, immunocompromised, and those with associated comorbidities or deranged liver function.
Severe acute respiratory syndromeThis was the first pandemic of the 21st century caused by a new strain of coronavirus (SARS-CoV1). It started in China in 2002 and later spread to Vietnam, Hong-Kong, Singapore, North America, Canada, and Europe. The disease affected around 8000 people and resulted in death in 10% cases, which prompted WHO to issue a global threat alert for this atypical pneumonia 25 Although the epidemic subsided in 2003 and no fresh cases have been reported, but there remains a constant threat of reappearance of the disease.
Mode of transmission is through direct contact via droplets or fomites that peaks in 2nd week of the disease. Its infectivity is evident by the fact that almost 22% of health care providers who came in contact with the patients acquired the infection 26 It is intriguing to note that apart from the respiratory secretions, virus was isolated in urine, feces and tears27,28 raising the possibility of alternate modes of spread.29 The likely access of virus into the eyes is through direct infection of the ocular surface, the spread of disease from upper respiratory pathway via the nasolacrimal duct, or by lacrimal gland infection through hematogenous spread. Ocular surface can also be the route of entry as conjunctiva and cornea possess angiotensin converting enzyme 2 (ACE2) that acts as receptor for SARS-CoV-1. But ACE2 receptors in the ocular surface are less abundant and have lower affinity for the virus in comparison to other tissues like lungs, kidneys, and gastrointestinal tract. 30,31
The shreds of evidence suggesting the presence of the virus in tear and conjunctiva are very conflicting. A study from Singapore detected SARS-CoV-1 from tears in 3 out of 8 confirmed cases 28 Conversely, a study from Hong Kong failed to isolate the virus either from tears or conjunctival cells of 17 confirmed cases, using both RT-PCR and viral cultures.32 Another longitudinal cross-sectional study from Singapore could not isolate the virus using RT-PCR in 126 conjunctival swabs taken weekly in 64 recovering patients of SARS.33 The inability to isolate the virus could be because the patients in this study were in the convalescent phase. Besides, ocular surface can still be a conduit of infection. Factors like low sensitivity of RT-PCR or improper timing of sample collection can very well influence the results in various studies. Based on the best available evidence, WHO has considered tears as one of the body fluids that may contain the virus. 29 Therefore even though there are no reports of any ocular manifestations of the virus, we cannot rule out the possibility of unprotected eyes as the possible route of entry or viral transmission. 34
Middle East respiratory syndrome MERS-CoVThe disease first erupted in Saudi Arabia in 2012 and since then has appeared intermittently in clusters in various countries. The outbreak is mostly associated with a history of travel to the Middle East. The hotspots of the epidemic were Riyadh, Jeddah, and South Korea. Until 2019, 2499 laboratory confirmed cases have been reported worldwide from 27 countries with 858 deaths 35 Although it was not as widespread as other pandemics it was known for severe respiratory disease and higher lethality with the reported death rate of 34%. 35 Unlike SARS-CoV1, MERS-CoV continues to circulate slowly in populations.
MERS-CoV is a novel coronavirus of zoonotic origin. The disease has been linked to the transmission through dromedary camels and bats. The primary mode of infection is believed to be via close contact with the animals and the consumption of raw animal products. Secondary cases then occur via human-to-human transmission through respiratory droplets and nosocomial transmission accounts for nearly half of the cases 36
As per the study conducted in 76 Omani dromedary camels, 5 cases (6.6%) showed high viral load in nasal as well as conjunctival swabs indicating that these sites can be possible routes of transmission 37 Despite these findings, ocular involvement remains a rare phenomenon in MERS-CoV with only 2% (6/261) cases reported to have conjunctivitis in a retrospective study conducted in the Makkah region of Saudi Arabia. 38
COVID 19/ SARS-CoV-2This is an ongoing pandemic that started in December 2019 as a cluster of cases with lower respiratory tract infection linked to the local seafood market in Wuhan, China. To date, there are approximately 40 million cases in over 215 nations with the death toll reaching 1.1 million. The disease has been declared as a Public Health Emergency by WHO 39
The exact animal source is still unknown, but pangolin and bats are considered to be the probable culprits 40,41 Its transmissibility is higher than the previous two strains because of higher reproducibility.42 Three primary modes of transmission identified are through droplets, close contact via fomites and aerosol. Other modes are being researched. As the virus has been detected in stool 43 and ocular surface, 44 we must keep in mind the possibility of transmission through these routes.
Apart from the systemic symptoms of respiratory discomfort and pneumonia 45 several reports have described ocular manifestations of the disease and in rare instances have been the presenting feature of the disease. 46–49 The role of eye in COVID-19 was brought under the spotlight after a leading Chinese respiratory expert hinted that unprotected eye probably resulted in infection in his case. He developed conjunctivitis days before other systemic features.50 Similar reports of acquiring conjunctivitis followed by flu manifestations in health personnel who did not use eye protection were seen. 48,49 All this evidence sums up in favor of eye being a likely portal of entry for the virus especially in a hospital setting where the health care worker comes in close contact with the patients. After gaining access to the ocular surface, the virus may either directly infect the eye via the ACE 2 receptors 51 (as seen in SARS-CoV-1) or enter the respiratory tract via the nasolacrimal duct. 52
A prospective interventional study that sampled the tears and conjunctival secretions in 30 patients obtained positive RT-PCR in one case who had conjunctivitis. The yield of the virus from conjunctival samples using PCR can be poor due to several factors as discussed in SARS- CoV-1. The only study that reports a fair amount of ocular involvement in SARS-CoV-2 is a retrospective study from China. They described conjunctival hyperemia, chemosis, epiphora, and increased secretions in 32% (12 of 38) of clinically confirmed cases of SARS-CoV-2, although the viral yield was poor from conjunctival swabs. Ocular symptoms were more common in patients with severe systemic disease 53 Other ocular manifestations described are keratoconjunctivitis, follicular conjunctivitis, hemorrhagic and pseudomembranous conjunctivitis particularly seen in patients with severe respiratory involvement. Pseudomembranous and hemorrhagic conjunctivitis has been reported in a patient 19 days after the onset of symptoms for which topical antibiotics and steroids were administered along with daily debridement of pseudomembranes to prevent conjunctival fibrosis. Complete resolution in this case occurred within a month. 54,55 WHO-China Joint Mission estimated the conjunctival involvement to be around 0.8% in SARS-CoV-2. 56 As per available literature, ocular manifestations may be seen in the range of 1.6% to 32%. 46,48,53,57 But it is important to be able to differentiate viral conjunctivitis from other forms of conjunctival involvement that are not uncommon in intensive care patient. 58 Recently, abducent nerve palsy and retinal involvement in the form of hyper-reflective lesions at the level of inner plexiform layer and ganglion cell layer (precursor of viral retinitis) have also been described. 49,59,60 Nevertheless, a general practitioner can miss the ocular findings and that may go unreported implying that the true incidence of ocular involvement may be underreported. As various antivirals are being tried in its management, there is also growing concern about the retinal toxicity related to some of these drugs like chloroquine and hydroxychloroquine 61
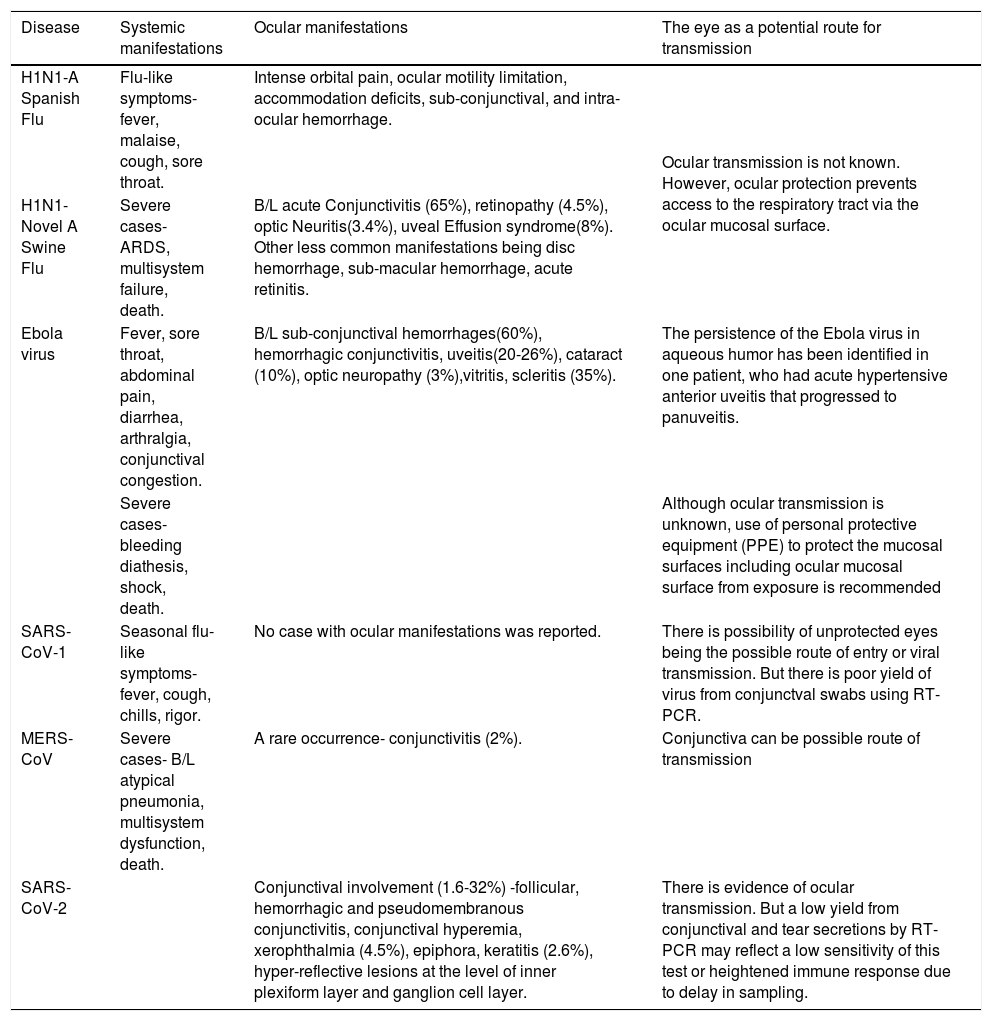
Table 1 summarizes the details of systemic and ocular manifestations of various pandemics discussed above.
Summary of systemic and ocular manifestations of recent pandemics.
| Disease | Systemic manifestations | Ocular manifestations | The eye as a potential route for transmission |
|---|---|---|---|
| H1N1-A Spanish Flu | Flu-like symptoms- fever, malaise, cough, sore throat. | Intense orbital pain, ocular motility limitation, accommodation deficits, sub-conjunctival, and intra-ocular hemorrhage. | Ocular transmission is not known. However, ocular protection prevents access to the respiratory tract via the ocular mucosal surface. |
| H1N1-Novel A Swine Flu | Severe cases- ARDS, multisystem failure, death. | B/L acute Conjunctivitis (65%), retinopathy (4.5%), optic Neuritis(3.4%), uveal Effusion syndrome(8%). Other less common manifestations being disc hemorrhage, sub-macular hemorrhage, acute retinitis. | |
| Ebola virus | Fever, sore throat, abdominal pain, diarrhea, arthralgia, conjunctival congestion. | B/L sub-conjunctival hemorrhages(60%), hemorrhagic conjunctivitis, uveitis(20-26%), cataract (10%), optic neuropathy (3%),vitritis, scleritis (35%). | The persistence of the Ebola virus in aqueous humor has been identified in one patient, who had acute hypertensive anterior uveitis that progressed to panuveitis. |
| Severe cases- bleeding diathesis, shock, death. | Although ocular transmission is unknown, use of personal protective equipment (PPE) to protect the mucosal surfaces including ocular mucosal surface from exposure is recommended | ||
| SARS-CoV-1 | Seasonal flu-like symptoms- fever, cough, chills, rigor. | No case with ocular manifestations was reported. | There is possibility of unprotected eyes being the possible route of entry or viral transmission. But there is poor yield of virus from conjunctval swabs using RT-PCR. |
| MERS-CoV | Severe cases- B/L atypical pneumonia, multisystem dysfunction, death. | A rare occurrence- conjunctivitis (2%). | Conjunctiva can be possible route of transmission |
| SARS-CoV-2 | Conjunctival involvement (1.6-32%) -follicular, hemorrhagic and pseudomembranous conjunctivitis, conjunctival hyperemia, xerophthalmia (4.5%), epiphora, keratitis (2.6%), hyper-reflective lesions at the level of inner plexiform layer and ganglion cell layer. | There is evidence of ocular transmission. But a low yield from conjunctival and tear secretions by RT-PCR may reflect a low sensitivity of this test or heightened immune response due to delay in sampling. |
In the wake of 2009 influenza pandemic, CDC had suggested certain guidelines for the protection of the health care personnel using a hierarchy model with key features of limiting the exposure to the disease, engineering control measures that includes modified work practices and protocols to eliminate the exposure at the source, administrative control measures and the use of personal protective equipment 62 This model may work equally well for other infectious diseases. Ophthalmologists and optometrists that form the frontline workforce in eye care need to implement these guidelines in clinical practice for the patient’s and their own safety in times of an epidemic.
Projecting to ophthalmology, we can minimize the exposure by limiting the outpatient visits. A triage of the diseases of respective sub-specialties should be designed and non-urgent procedures like refraction, cataract, refractive and squint surgeries in adults should be put on hold. The telemedicine model of clinical care can be used and only the patients who need urgent attention should be seen physically. Unless an emergency, the postponement of appointment should be considered for those showing suspected symptoms of the disease. Patient stratification should be done with a targeted approach to manage ocular emergencies as a priority such as trauma, ocular infections, acute angle-closure glaucoma, phacomorphic or neonatal glaucoma, congenital cataract, retinal arterial and venous occlusions, retinal detachments, uveitis, optic neuritis, compressive or traumatic optic neuropathy and other entities that can lead to irreversible vision loss 63–65
Screening of all patients should be done at the entry of the premises and those showing suspected symptoms may be examined in a separate station or called later after the resolution of symptoms. Necessary modifications in work practices and protocols must be strictly adhered to that includes obtaining a specific detailed history of recent international travel, contact with a confirmed case, and any disease specific symptoms. The management and employers have a key role at this level as the staff deputation has to be changed in the face of the epidemic. A dedicated team of health care workers should be assigned for the care of confirmed cases that works for a pre-defined tenure and are later replaced by the next team. The health care workers showing any signs of affection should be timely tested and isolated. Utmost care should be taken for the safety, hygiene and welfare of the staff.
The judicious use of Personal Protective Equipment will not only aid in protecting the health care forces but will also keep a check on the cross-transmission. As the evaluation techniques demand close physical proximity with the patient’s eyes and face, the ophthalmologists and the optometrists are quite predisposed to acquire infection. Hence, it is paramount to be adequately trained and well acquainted with the preventive strategies before approaching any patient. Use of eye goggles, facemasks, face shields, head caps, gloves, and protective gear has been specifically advocated 63–67 While PPE can protect from exposure, its effectiveness is subject to several factors such as adherence to the above-cited measures. One must observe proper hand etiquettes before and after examining the patient and avoid any unnecessary test. To prevent the respiratory and droplet transmission, the installation of previously used radiographs as protective shields on the slit lamp to separate the viewing arm from the patients’ end has been advised (Fig. 3). Disinfection of equipment should be done using 70% ethyl alcohol especially forehead rest, chin rest and knobs of slit lamp, autorefractometer, keratometer, tonometer and topographer after every use. Devices that come in direct contact with the patient like trial frame, trial lenses, applanation prism, ultrasound probe, UBM probe, iridotomy and laser photocoagulation lenses need to be appropriately disinfected. Direct ophthalmoscopy and contact procedures such as Goldman tonometry should be deferred wherever possible 64,67,68 Perimetry using enclosed screen rather than bowl type has been recommended. 69 Although contact lens wear is considered safe during this pandemic, 70 reinforcement of contact lens care and maintenance should be advised using specific contact lens care solutions. Paediatric examination becomes especially challenging as they may not wear a mask or adhere to other safety guidelines. Apart from the precautions discussed above, use non-contact occlusion in children and avoid touching them during the examination. As many of these viral infections may present as conjunctivitis an ophthalmologist and optometrist should take sufficient precaution while examining such patients. They should not be evaluated barehanded and gloves used should be properly discarded after examining each patient.
Amongst the health workers, the high-risk groups like pregnant females, elderly and those with chronic diseases like diabetes, asthma and heart ailments should be highly vigilant and report even slightest of symptoms. While numerous candidate vaccines are at various stages of development, it is prudent to follow guidelines that prevent inter personal spread like social distancing, masks and frequent hand-washing till any definitive treatment is available.
ConclusionThe ocular manifestations of various viral pandemics elaborated in this article depict the key role of ophthalmologists in such hazardous situations. As many of these pathogens are shed in the ocular secretions and some have even been isolated in various ocular tissues, ocular features might be the only presenting symptom of the underlying disease. Therefore, being the first point of contact an ophthalmologist needs to act with ample preparedness and responsibility. Also, as the conventional ophthalmological examination techniques require proximity to the eyes and face of the patients, modifying the current practice protocols in the wake of the ongoing epidemic becomes imperative. Tele-ophthalmology, though presently in its naïve state, can help the modern-day practitioners by aiding in the triage of the patients based on the history and thereby minimizing the exposure. The frontline health care providers are the most exposed to acquire and transmit such diseases. Therefore, during such adversity a strict implementation of the preventive practices is the only way forward.
FundingNone.
Conflict of interestThe authors have no conflicts of interest to declare.
I would like to acknowledge Mr. Vinay Gupta, BSc Optometry, for his contribution in designing the figures.