To study long-term results of MyoRing treatment of keratoconus.
MethodsRetrospective study of MyoRing implantation into a corneal pocket for keratoconus.
ResultsCorneal thickness at the thinnest point remained unchanged, SIM K's, manifest sphere and cylinder were significantly improved at the first follow-up 9 months postoperatively and remained stable until the last follow-up about 5 years after surgery. Uncorrected and corrected distance visual acuity (UDVA, CDVA) were significantly improved at the first follow-up 9 months postoperatively and were further ameliorated until the last follow-up about 5 years after surgery.
ConclusionThe treatment was safe and effective with continuing improvement of visual acuity during the 5 years after surgery.
Estudiar los resultados a largo plazo del tratamiento del queratocono con MyoRing.
MétodoEstudio retrospectivo de la implantación del MyoRing en un bolsillo corneal en los casos de queratocono.
ResultadosEl espesor de la córnea en el punto más fino no reflejó cambios, mejorando los valores de SIM K, esfera y cilindro manifiesto durante el seguimiento a los 9 meses de la intervención y permaneciendo entonces estables hasta el último seguimiento a los 5 años de la misma. La agudeza visual no corregida y corregida (UDVA, CDVA) mejoró considerablemente a los 9 meses de la intervención, y continuaron mejorando significativamente hasta el último seguimiento a los 5 años de la misma.
ConclusiónEl tratamiento resultó seguro y efectivo, con mejora continua de la agudeza visual durante los 5 años posteriores a la cirugía.
Keratoconus is a rare disease which is characterized by progressive steepening and thinning of the cornea, thus resulting in progressive vision loss.1 Corneal intrastromal implantation surgery (CISIS) with MyoRing implantation has demonstrated to be an effective and safe treatment for visual rehabilitation in myopia and keratoconus.2,3 This paper presents 5 years follow-up data of MyoRing treatment for keratoconus of a central European population, discusses the related treatment principles and provides a guideline for evaluating treatment success.
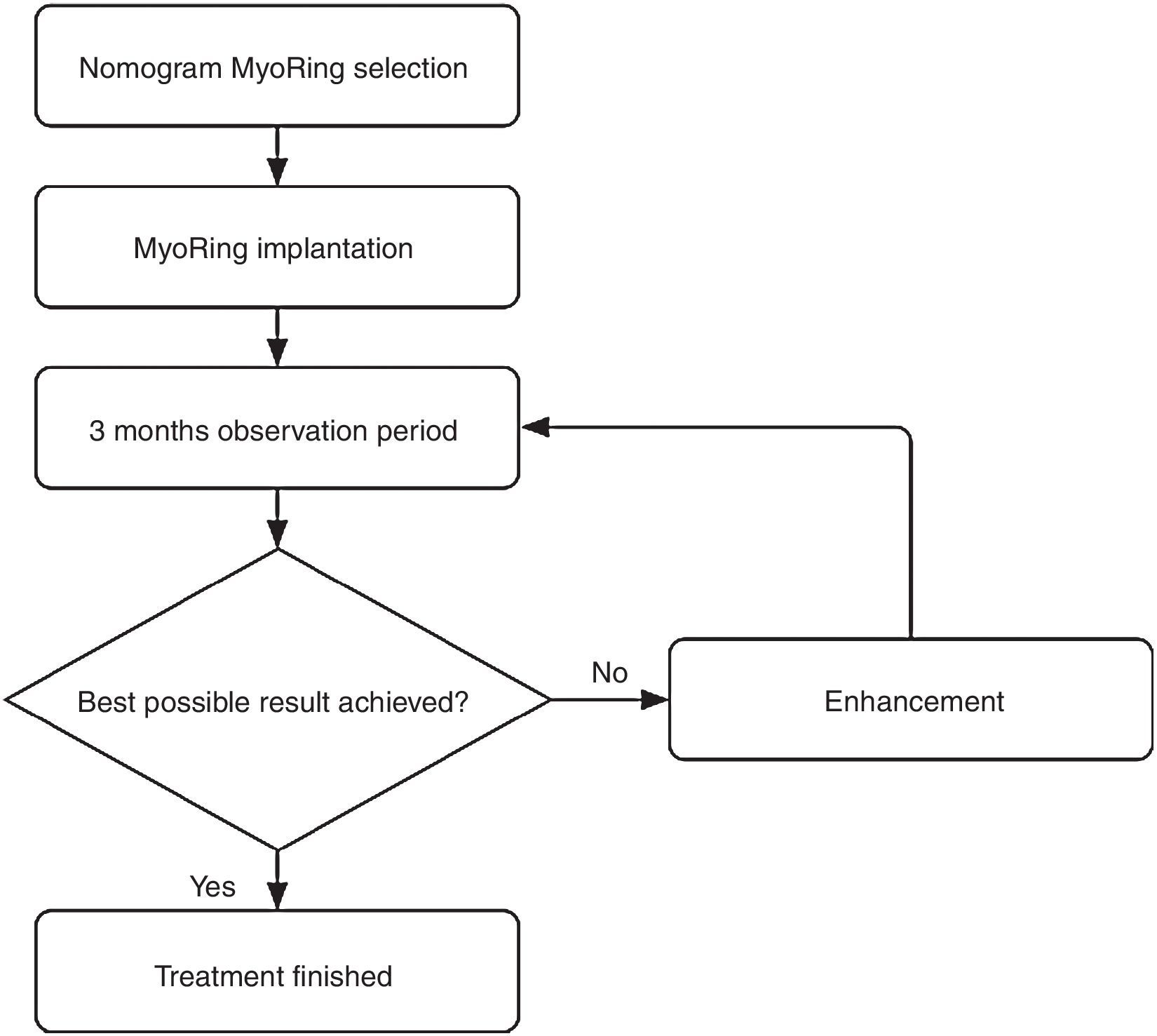
Materials and methodsAs described elsewhere,2,3 CISIS starts with the creation of an intrastromal corneal pocket of 9mm in diameter at a depth of 300microns by means of the PocketMaker Ultrakeratome (DIOPTEX GmbH, Austria), followed by the implantation of the MyoRing (DIOPTEX GmbH, Austria) via a small lamellar tunnel of less than 5.5mm using a particular implantation forceps. The MyoRing finally has to be centered by using the real postoperative optical axis as a reference. The lamellar tunnel is self-sealing and requires no suture. The procedure is minimally invasive, causes no pre- or postoperative pain and will take only 10min when performed by a trained and experienced surgeon.
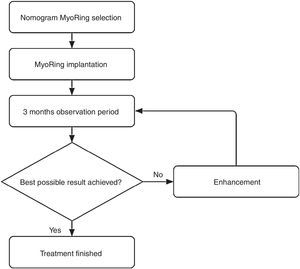
Postoperatively, the eye requires neither bandage lenses nor patching. The patient is advised to apply a combination of steroid and antibiotic eye drops hourly until undergoing the first follow-up exam on the first postoperative day. Between this first postoperative exam until two week after surgery, the patient is advised to reduce the application of the aforementioned combination of eye drops to merely 5 times a day. Thereafter no further medical therapy is required. The next follow-up exam is usually performed 3 months after surgery to evaluate whether the result is already optimal or may be further enhanced. This is called the initial postoperative observation period.
According to the suggested visual potential of the individual eye, some 20% of the patients draw a visual benefit from a simple postoperative enhancement.4 The enhancement is performed either by optimizing the position in relation to the real postoperative optical axis or by exchanging the MyoRing for one with different dimensions. An enhancement is accomplished easily and takes usually less than one minute without causing the patient intra- or postoperative pain.
This iterative optimization procedure is based on the rationale that an a priori clinical determination of all information required for a nomogram that predicts the initial result in 100% of the cases is simply impossible in the in vivo corneal system. In other words, complex nomograms with many decision criteria suggest a nomogram accuracy to the user which is completely unreal. In the case of MyoRing treatment, the accuracy of the nomogram therefore reflects the real accuracy of the maximum possible degree of determination of the corneal system. This results in some 20% of patients who benefit from a simple enhancement 3 months after initial surgery. In other words, if the prediction accuracy of a system is 80% for the initial approach, it only takes a simple, short and safe procedure for further enhancement in a second step to achieve a success rate of nearly 100%. This is exactly the philosophy behind the MyoRing treatment.4
In this retrospective study, two postoperative follow-ups per patient have been included: the first one approximately one year after the last surgical intervention and the second one approximately 5 years later. The examination performed at the end of both follow-up periods used for the study included Scheimpflug measurement for topography, pachymetry at the thinnest point (ct) and K-readings SIM K1, SIM K2 and K=(SIM K1+SIM K2)/2 using the Pentacam (Oculus GmbH, Germany), uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA). Visual acuity data are represented in logMAR.
Statistical evaluation was performed using the two-tailed paired t-test for comparison of data having Gaussian distribution. The normality testing for Gaussian distribution was performed using the D’Agostino & Pearson omnibus normality test. In the case of non-Gaussian data distribution, the data were compared using the Wilcoxon matched-pairs signed rank test. To qualify data as being significantly different in statistical terms, a p-value of less than 0.05 was used. The statistical data are presented in mean±standard error (se) in the case of Gaussian distribution and as median and range in case the normality test was not passed.
Results53 eyes of patients pertaining to a Central European population from all over Austria and Germany were treated within the selected period. A subgroup of 17 eyes of 13 patients fulfilled the criteria of having 2 independent follow-up exams within the selected follow-up periods with all required data available (corneal thickness, topography including K-readings, manifest refraction, uncorrected distance visual acuity UDVA, corrected distance visual acuity CDVA). Many patients had to cover a long distance of sometimes more than 1000km for examination in our center. The majority of that patients refused to spend two days and a flight for long-term follow-up examination when they were happy with the results. The study had to be limited to patients representing a homogenous ethnic group (central European population) to rule out an influence resulting from ethnic variations. The nomograms of patients from the Middle East, for example, must be different from those used for European populations to avoid overcorrections.4 The first follow-up exam performed in the eyes included in the study was between 3 months and 24 months after surgery (9±1.8). The second follow-up exam was between 36 months and 90 months after surgery (56±3.9). 10 eyes were right eyes (OD) and 7 were left eyes (OS). Two patients (4 eyes) were female and 11 patients (13 eyes) were male. The age of the patients at the time of surgery ranged from 21 to 50 years (median 35 years). Of the 17 eyes 3 (17.5%) had grade I, 4 (23%) had grade II, 5 (30%) had grade III, 3 (17.5%) had grade IV and 2 (12%) had grade V according to the grading after Alio et al.5 A minimum of 3 eyes (17%) experienced progression of the disease in the year prior to surgery. Four of the 17 eyes (23.5%) had an enhancement intervention 3 months after the initial treatment, during which the implant was replaced by a stronger or weaker one. For these 4 eyes, the postoperative follow-up period started with the date when the enhancement was performed. No eye underwent more than one enhancement procedure. All surgeries were performed by one surgeon (A.D.) and did not result in any intra- or postoperative complications.
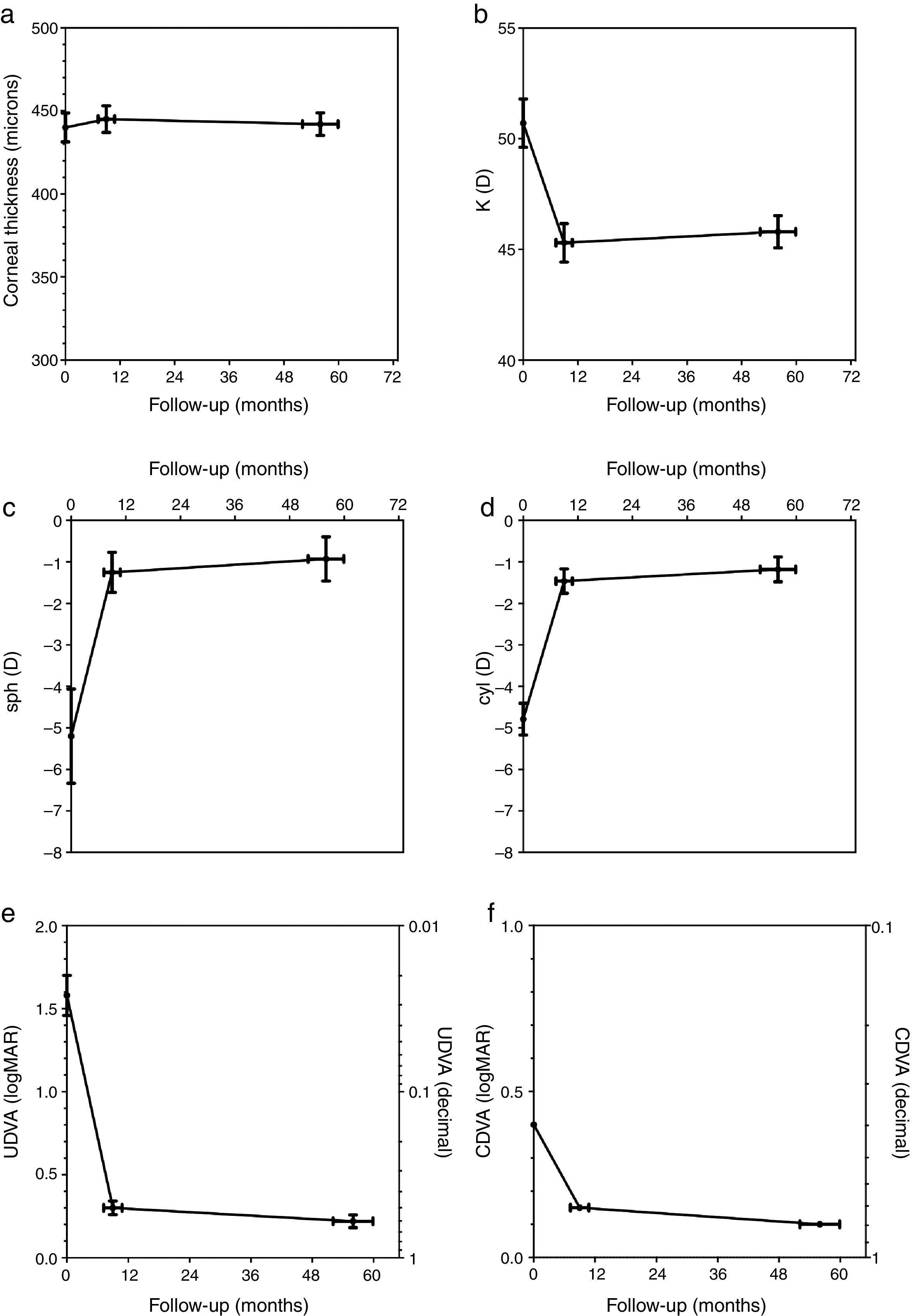
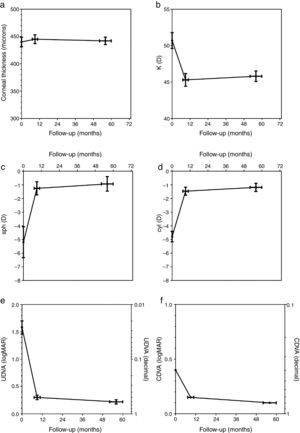
The corneal thickness measured at the thinnest point showed no statistically significant changes at both follow-ups compared to the preoperative value or compared to the other postoperative value. Fig. 1a shows the graphical representation. The values for corneal thickness were 440±9 microns preoperatively, 445±8 microns at the first follow-up and 442±7 microns at the second follow-up, respectively.
The average central K-value determined by K=(SIM K1+SIM K2)/2 was significantly lower than the preoperative value of 50.68±1.1 diopters (D) at both follow-ups. No significant changes occurred between the first (45.27±0.88 D) and second postoperative follow-up (45.76±0.74 D). Fig. 1b shows the time course of K.
The manifest sphere (sph) as well as the cylinder (cyl) were significantly reduced at both follow-ups compared to the preoperative value (sph −5.21±1.13D, cyl −4.79±0.39D). However, no significant change was observed between the first postoperative follow-up (sph −1.25±0.48D, cyl −1.46±0.30D) and the second follow-up (sph −0.93±0.52D, cyl −1.18±0.31D) (Fig. 1c and d).
Uncorrected distance visual acuity (UDVA) improved significantly between the preoperative exam (1.58±0.38logMAR) and the first postoperative follow-up (0.30±0.041logMAR), as was the case between the preoperative exam and the second postoperative follow-up (0.22±0.038logMAR). UDVA also improved significantly between both postoperative follow-up examinations. Fig. 1e shows the time course of UDVA, demonstrating an improvement of some 12 lines on average. All eyes gained lines at both postoperative follow-ups when compared to the preoperative state.
Corrected distance visual acuity (CDVA) had no Gaussian distribution in the preoperative exam nor after both postoperative follow-up periods because the average natural limit for improvement at 20/20 (0.0logMAR) is closer to the measured CDVA values when compared to UDVA. The preoperative median of CDVA was 0.4logMAR (range 0.22–1.0). The median of CDVA at the first follow-up was 0.15 (range 0.0–0.52),and at the second follow-up 0.1 (range 0.0–0.4), respectively. CDVA was statistically different in all 3 examinations. A particular improvement of CDVA was observed between the preoperative exam and the first postoperative follow-up; a further significant improvement was seen between the first and second postoperative follow-up. The related graph in Fig. 1f demonstrates an improvement of approximately 2–3 lines on average. All eyes gained lines and no eye lost lines in the two postoperative follow-ups compared to the preoperative state.
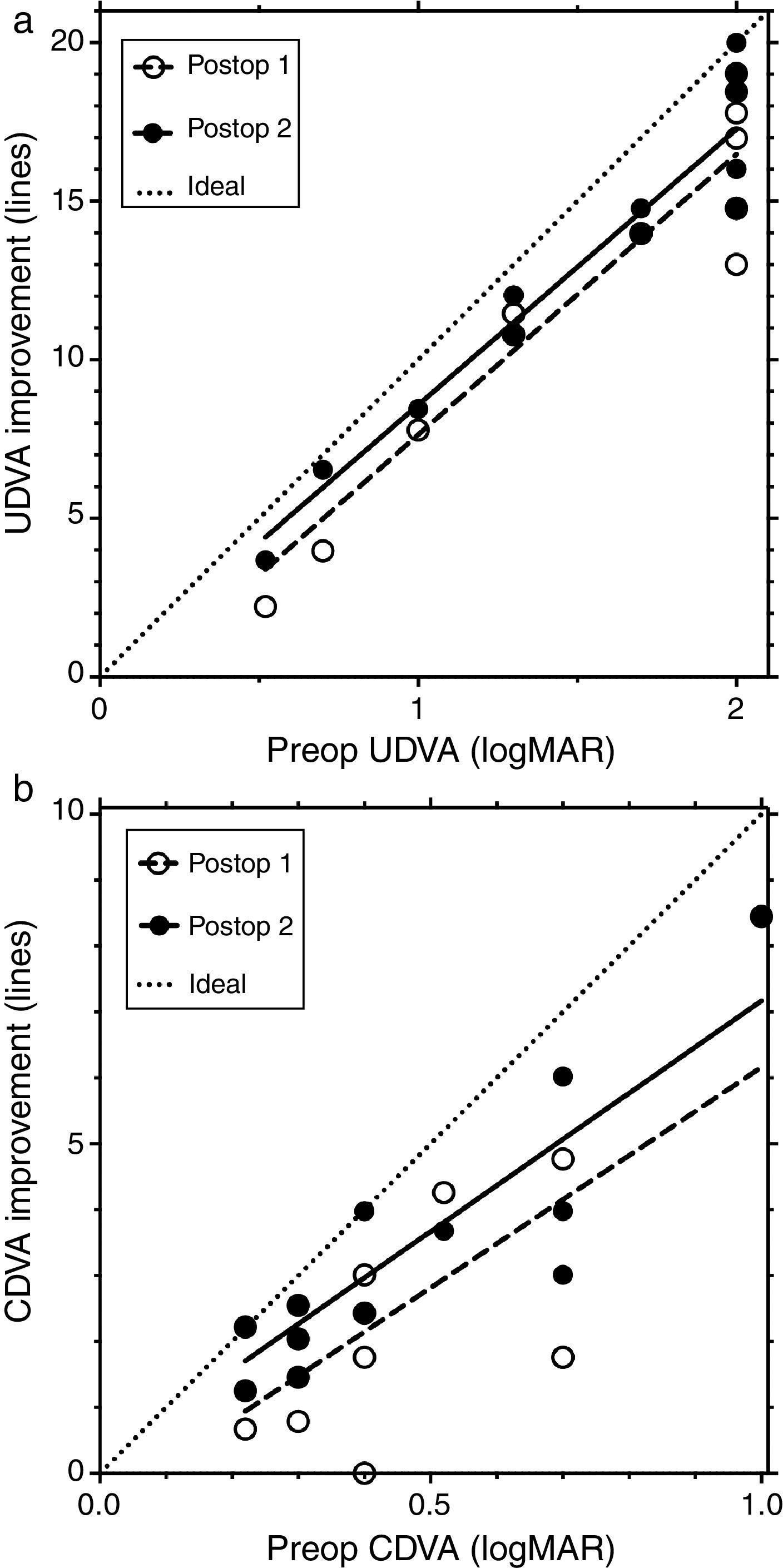
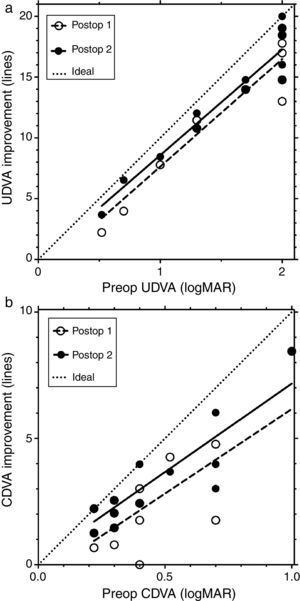
Fig. 2 shows the line improvement of uncorrected and corrected distance visual acuity as a function of the severity of the disease measured as preoperative visual acuity. This makes sense as the potential for improvement is different for mild and advanced cases; mild cases require fewer lines to reach 20/20 than advanced ones. The same is true when comparing UDVA and CDVA: the improvement potential for CDVA is less than for UDVA.
Fig. 2a shows the line improvement of UDVA at first and second follow-up compared to preoperative UDVA as a function of preoperative UDVA. Fig. 2b shows the line improvement of CDVA at first and second follow-up compared to preoperative CDVA as a function of preoperative CDVA. The dashed lines are the result of the linear regression of line improvement in UDVA (r2=0.8865, y=8.844x−1.21, Fig. 2a) and CDVA (r2=0.5591, y=6.69x−0.53, Fig. 2b) at the first postoperative follow-up (open circles). The solid lines are the result of the linear regression of the line improvement of UDVA (r2=0.8992, y=8.691x−0.114, Fig. 2a) and CDVA (r2=0.7588, y=7.0x+0.17, Fig. 2b) at the second postoperative follow-up (black circles). Some data points are overlaid by others in Fig. 2. The dotted lines represent the maximum possible (ideal) improvement to 20/20 visual acuity. In UDVA and particularly in CDVA, visual acuity additionally improves between the first and second follow-up; this improvement is indicated by a shift of the linear regression line toward the ideal (dotted) line.
DiscussionThe results of the present study show a significant improvement of K-reading, sphere and cylinder as well as UDVA and CDVA after MyoRing implantation in keratoconus at the first follow-up approximately 9 months after surgery. While K-reading, sphere and cylinder remain unchanged between the first and the second follow-up around 5 years after surgery, UDVA and CDVA show a continued statistically significant improvement during this second postoperative period (Figs. 1 and 2).
The statistically significant improvement of UDVA and CDVA even during the long-term postoperative period not only indicates a significant degree of visual rehabilitation but may also indicate a stabilization of the diseased cornea following MyoRing implantation. Moreover, the continuous improvement in CDVA during the postoperative course in all grades of the disease (Fig. 2) points at an ongoing postoperative “regularization process” of the diseased cornea after treatment. The average visual improvement of 12 lines and more in UDVA and 3 lines in CDVA correlates with earlier reports which consider one-year follow-ups after treatment.3 This data is in striking contrast to the data obtained from ring segments, which hardly reach comparable improvements in visual acuity.6–8
Ring segments have the disadvantage of resulting in a long-term postoperative loss of visual acuity.9,10 MyoRing implantation, by contrast, stabilizes the corneal thickness even in previously progressive cases, as several reports indicate.11–13 None of the 3 eyes (17%) in the present study that had documented progression during the year prior to treatment did so after MyoRing insertion. However, since the keratoconic cornea of an individual patient can only be characterized as progressive through a retrospective analysis, it is generally impossible to qualify such a cornea as progressive. In other words, if a cornea was progressive in the recent past but is no longer progressive after treatment, there may be two possibilities: either the past progression and future halting of progression is the natural course of the disease or the treatment is effective and stops progression. Another possibility is that the disease is not progressive at a certain point of time but becomes progressive in the future. Therefore, the efficacy of a therapy in halting keratoconus progression cannot be evaluated by simply comparing two groups of cases in terms of their past individual behavior: progressive and non-progressive. Moreover, it is necessary to compare a treated group with a similar group not undergoing treatment, and to relate the number of patients that would have progressed during the follow-up period without treatment to those who progressed after treatment. Longitudinal studies show a progression rate of approximately 25% in patients with an average age of 25 years suffering from mild to moderate keratoconus.14 Considering that the population included in the present study has a higher average age (35 years) and the percentage of documented cases showing progression immediately prior to treatment was 17%, it can be estimated that approximately 20% of the eyes would have progressed during the average follow-up period of 5 years without treatment. The estimation is based on the assumption that at least one eye out of the preoperatively not progressing eyes is likely to progress during the 5 year observation period but counting it just with 50% probability.
The fact that no significant progression was observed in the present study after MyoRing treatment during an average follow-up period of some 5 years is no total proof that MyoRing implantation stops progression, but it is a very strong indicator. One explanation for the stop of progression of keratoconus after MyoRing implantation may be found in the biomechanics of the cornea before and after MyoRing implantation.15 Since the MyoRing is a continuous full ring implant, with no disruption of continuity along its circumference, it acts in the cornea like a second (artificial) limbus and supports the cornea biomechanically in much the same way as a ceiling beam supports the ceiling of a room under load by separating the ceiling (cornea) into two compartments and reducing the load on each compartment.15 Specifically, the MyoRing is able to take up a significant amount of the load acting on the cornea. This mechanism may result in a strengthening of the cornea by a factor of 3 after MyoRing implantation, which corresponds to an increase in effective corneal thickness of a factor of 3. A discontinuous ceiling beam (in analogy to ring segments), in turn, is unable to stabilize a ceiling under load.15
Another important difference between ring segments and the MyoRing is that the MyoRing provides the surgeon with all three possible degrees of freedom to achieve the optimal result in any given case (implant thickness, implant diameter and implant position), whereas ring segments provide only one degree of freedom (implant thickness).16 Specifically, the position of the segment is determined by the preparation of the circular tunnel relating to the preoperative fixation (optical axis), which in turn depends on the preoperative corneal shape. In this context, it is important to understand that the misalignment of an implant by about 0.5mm may have a dramatic impact on the visual results.17 The MyoRing is placed inside a corneal pocket with a diameter of 9mm, which allows to align the implant to the postoperative fixation of the eye at the end of the surgical intervention. This may be a further reason why MyoRing implantation outperforms ring segments in terms of vision improvement.
To be able to exploit the full potential of the MyoRing, it is also important to understand the natural boundaries of a nomogram in predicting the perfect implant dimension in any specific situation and to draw the right conclusion from this understanding. While the nomogram for the MyoRing treatment of keratoconus is very simple (it depends only on the average SIM K), the nomograms for ring segments tend to be much more complex yet often do not allow a satisfactory prediction of the results.17,18 It seems that ring segment nomograms overestimate the biomechanical predictability for keratoconic corneas in vivo. MyoRing treatment pursues a different strategy than ring segments and tries to avoid pseudo-accuracy in the nomogram. The rationale of MyoRing treatment is: If a system cannot be predicted preoperatively with 100% accuracy, the surgical approach has to be designed in such a way that it becomes extremely simple to enhance the result in a certain percentage of cases postoperatively, and this enhancement procedure is then incorporated into the treatment strategy. Consequently, MyoRing treatment is based on a lamellar surgical concept focusing on a small lamellar incision that does not require suturing. The replacement of a MyoRing implant is a one-minute job which causes no pain or irritation. Clinical problems during or after MyoRing exchange have not been observed. The costs for exchanging the implant after 3 months is covered by the initial payment of the patient – so the patient do not have to pay extra for that. In the present study, 4 out of 17 MyoRings (23.5%) were replaced after the initial postoperative observation period of three months to achieve optimal individual results. The results are independent of cone position (central or non-central) because the MyoRing inside a corneal pocket automatically “corrects” the corneal area in the right way.19
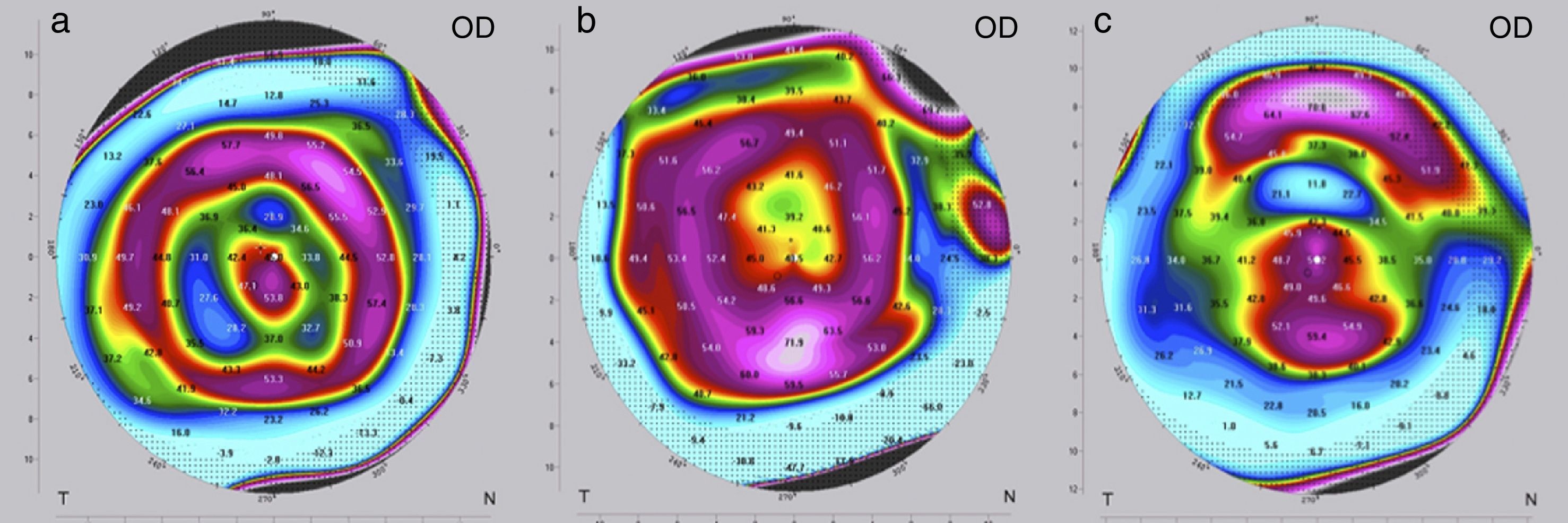
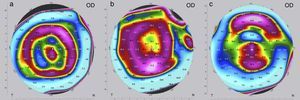
The criteria for optimization, i.e. the need to change the implant for a “stronger” or “weaker” one, may differ depending on the following situations. If visual acuity (UDVA, CDVA) at the end of the postoperative observation period of three months is as preoperatively planned, no further intervention is needed and treatment is completed. In these cases, the refraction (spherical equivalent SE) is normally close to zero and the topography shows a certain characteristic pattern in the postoperative tangential map (Fig. 3a); the color-marked local corneal curvature has the shape of a “central island” and the ring-shaped topography of the MyoRing is of “homogeneous” intensity (local curvature). If visual acuity at the end of the postoperative observation period of three months does not match preoperative expectations, refraction and topography will have to play a role in further decision making. In the case of overcorrection, the refraction shows a hyperopic shift and the color-marked “intensity” of the concentric ring in the tangential map is most pronounced over the “central island” (Fig. 3b). In this case, the MyoRing has to be replaced by a “weaker” one. The opposite applies in the case of undercorrection. Here the spherical equivalent is usually in the myopic range, and the prominent feature in the tangential map is the “central island”, with the concentric ring pattern being clearly underrepresented (Fig. 3c). This is an indication to replace the implant by a “stronger” one. “Weaker” means that the implant has a larger diameter and/or is thinner, “stronger” means the opposite. The rationale of MyoRing treatment of keratoconus for achieving optimal results is somehow related to hard contact lens fitting. But instead of analyzing fluorescein patterns as in contact lens fitting, the MyoRing is optimally adjusted to match the tangential topography map.
As sometimes seen in MyoRing-treatment videos (e.g. on YouTube), another mistake is to “mark” the cornea prior to implantation for the purpose of aligning the MyoRing. This procedure does not allow to achieve the best possible result in each individual case because optimal treatment requires alignment to the real postoperative fixation. The best results are achieved when the surgical microscope has a concentric light-source which the patient can fixate. At the end of the surgical intervention, once the MyoRing has been implanted into the corneal pocket, the patient is asked to fixate this light source. Then the MyoRing is shifted to its “central” position inside the pocket (between the center of the pupil and the central spot between the first and the second Purkinje Reflex of the concentric microscope light source). This procedure also helps to minimize the paralaxis error and to properly determine the center of the pupil.
To be able to assess the individual results of MyoRing treatment achieved by a specific surgeon, it is necessary to use a plot like the one in Fig. 2 allows to analyze the results, including the surgeon's specific performance, in detail. This performance can be assessed for mild, moderate and advanced cases (Fig. 4).
In conclusion, the MyoRing – when used according to the treatment principles described above – has the potential to produce excellent long-term vision results in mild, moderate and advanced keratoconus cases, regardless of cone position and disease progression.
Conflicts of interestDr. Daxer has an investment interest in DIOPTEX GmbH, Dr. Ettl and Dr. Hörantner do not have a conflict of interest.
The study was partly supported by the Austrian Research Fund (FFG). Dr. Daxer has an investment interest in DIOPTEX GmbH, Dr. Ettl and Dr. Hörantner have no financial interest. Preliminary results were presented at the Keratoconus Expert Meeting held during the Annual Meetings of the ESCRS 2014 in London and 2015 in Barcelona.