To compare femtosecond laser-assisted sub-Bowman keratomileusis (FSBK) versus laser-assisted subepithelial keratomileusis (LASEK) to correct moderate to high myopic astigmatism.
MethodsRetrospective, nonrandomized, interventional, comparative case series. A total of eight hundred and fifty-two eyes with myopic astigmatism of −1.5 diopters (D) or higher were included in the study. We compared 427 eyes treated with FSBK versus 425 eyes treated with LASEK with or without mitomycin C. Visual and refractive results were evaluated 1 day, 1 week, 3 and 6 months postoperatively.
ResultsSix months postoperatively, the residual spherical defect was slightly but significantly higher in the LASEK group (+0.15±0.62D) than in the FSBK group (+0.09±0.35D) (P=0.05). The postoperative residual astigmatism was also slightly but significantly higher in the LASEK group (−0.38±0.52D) than in the FSBK group (−0.26±0.45D) (P=0.0005). No significant differences were found in the efficacy (0.98±0.17 versus 0.98±0.36, P=0.6) and safety indexes (1.04±0.16 versus 1.05±0.37, P=0.1) between FSBK and LASEK. The enhancement rate was significantly higher in the FSBK group (22.6%) than in the LASEK group (15.5%) (P=0.01).
ConclusionsBoth FSBK and LASEK are safe and effective procedures to correct moderate to high myopic astigmatism. Slightly better visual and refractive results were observed in FSBK-treated eyes in a 6-month follow-up.
Comparar la queratomileusis sub-Bowman asistida por láser de femtosegundo (FSBK) y la queratomileusis sub-epitelial asistida por láser (LASEK) para corregir el astigmatismo miópico de moderado a elevado.
MétodosSeries de casos retrospectivas, no aleatorizadas, intervencionistas y comparativas. Se incluyó en el estudio a un total de ochocientos cincuenta y dos ojos con astigmatismo miópico de -1,5 dioptrías (D), o valores superiores. Comparamos 427 ojos tratados con FSBK frente a 425 ojos tratados con LASEK, con o sin mitomicina C. Se compararon postoperatoriamente los resultados visuales y refractivos al cabo de un día, una semana, tres y seis meses.
ResultadosTranscurridos seis meses de la operación, el defecto esférico residual fue ligera aunque significativamente superior en el grupo LASEK (+0,15±0,62D) en comparación al grupo FSBK (+0,09±0,35D) (P=0,05). El astigmatismo residual postoperatorio fue también ligera aunque significativamente superior en el grupo LASEK (-0,38±0,52D) en comparación al grupo FSBK (-0,26±0,45D) (P=0,0005). No se hallaron diferencias significativas en relación a los índices de eficacia (0,98±0,17 vs 0,98± 0,36, P=0,6) y seguridad (1,04±0,16 Vs 1,05±0,37, P=0,1) entre FSBK y LASEK. La tasa de retratamiento fue significativamente más elevada en el grupo FSBK (22,6%) que en el grupo LASEK (15,5%) (P=0,01).
ConclusionesTanto FSBK como LASEK resultan procedimientos seguros y eficaces en la corrección del astigmatismo miópico de moderado a elevado. Durante el seguimiento a seis meses en los ojos tratados con FSBK se observaron mejores resultados visuales y refractivos.
Laser in situ keratomileusis (LASIK) is a safe and effective procedure to correct several degrees of myopia.1 However, it is well accepted that surgical correction of moderate to high astigmatism seems to be less predictable than sphere correction.2,3 In addition, high astigmatism could be considered as a risk factor for the development of postoperative corneal ectasia. In fact, Randleman et al.4 found that the presence of a preoperative abnormal topography was the most significant risk factor for post-LASIK corneal ectasia, whereas Khalid et al.5 included the presence of an oblique cylinder greater than 1.5 diopters (D) as a risk parameter in their preoperative grading system for the detection of risk of corneal ectasia after LASIK.
Excimer laser surface ablation (SA) procedures, such as photorefractive keratectomy (PRK) and laser-assisted sub-epithelial keratomileusis (LASEK), have become the technique of choice in patients with thin central corneal thickness, those at risk for trauma, and those with corneal surface problems such as dry eye, recurrent erosion syndrome, or basement membrane disease.6 The fact that SA seems to be safe when performed on thin corneas7,8 and that the incidence of postoperative ectasia seems to be much lower after SA than after LASIK7,8 suggests that SA has less biomechanical impact on the cornea than LASIK.9 Femtosecond laser-assisted sub-Bowman keratomileusis (FSBK) shares with the mechanical LASIK the advantage of the fast visual rehabilitation, the painless postoperative period, and the low-risk of haze. In addition, it creates a predictable thin and planar corneal flap10,11 that is thought to have less biomechanical impact on the cornea that the thicker, meniscus-shaped flap obtained with a mechanical microkeratome.11 This is thought to bring FSBK closer to SA in terms of safety as far as corneal biomechanics is concerned.12
To our knowledge, only one study has been specifically designed to compare the visual and refractive outcomes of LASIK (performed with a mechanical microkeratome) versus SA for the correction of high astigmatism.13 Given the fact that FSBK has been proposed as an alternative to SA due to less impact on corneal stability than LASIK while maintaining LASIK's advantages, we decided to compare the visual and refractive results of FSBK versus SA to correct moderate to high astigmatism.
Materials and methodsWe performed a retrospective study of consecutive patients who had been operated with FSBK or LASEK using mitomycin C (MMC) when needed, to correct myopic astigmatism of −1.50D or higher. We excluded patients with unstable refraction, previous ocular surgery, topographic findings of keratoconus, ocular disease, and systemic disease that could interfere with wound-healing process such as diabetes mellitus and connective tissue disorders. The decision to perform LASEK instead of FSBK was based either on the calculated residual stromal thickness being too thin to perform FSBK (thinner than 300- to 250-μm) or on patient preferences after being fully informed about the advantages and disadvantages of both procedures. All patients signed the informed consent and the institutional review board approval was obtained.
Every patient underwent a full ophthalmologic examination before surgery including measurement of uncorrected visual acuity (UCVA), best spectacle-corrected visual acuity (BSCVA) (using a Snellen chart [Nidek auto chart projector CP 670; Nidek Co., Ltd., Gamagori, Japan] including manifest and cycloplegic refractions), slit-lamp biomicroscopy, tonometry (CT-80; Topcon, Tokyo, Japan), corneal pachymetry (Ocuscan Pachymeter RxP, Alcon Laboratories, Fort Worth, USA), keratometry and corneal topography (CSO; Compagnia Strumenti Oftalmici, Firenze, Italy), mesopic pupil measurement (Colvard pupilometer; Oasis, Glendora, CA, USA), and funduscopy.
Surgical techniqueAll FSBK and LASEK procedures were performed by two experienced surgeons (M.A.T. and M.G.-G.) using the same Esiris Schwind excimer laser (Schwind Eye Tech Solutions, Kleinostheim, Germany), a LASIK nomogram (in FSBK cases) and a PRK nomogram (in LASEK cases), and a conventional, non-wavefront or topography-guided, sphero-cylindrical treatment. All surgeries were performed under topical anesthesia (Lidocaine 2%; Xilocaine; APP Pharmaceutical, Schaumburg, IL, USA).
In the FSBK group, the femtosecond laser used was the IntraLase femtosecond laser (IntraLase Corp, Irvine, CA, USA) with the 60-kHz software, with the following parameters: a raster pattern using an energy level of 0.9μm, a side-cut energy of 0.9μJ, a 70° side cut angle, a hinge angle of 50°, an attempted flap thickness of 100μm and a flap diameter of 9mm. The flap was lifted with a spatula, the excimer laser ablation performed, the stroma was then washed with balanced salt solution (BSS) and the flap was put back in place. At the end of the surgery, antibiotic drops (ofloxacin 0.3%; Exocin; Allergan SA, Madrid, Spain) and nonsteroidal antiinflammatory drops (ketorolac trometamol 5mg/mL; Acular; Allergan SA, Madrid, Spain) were applied.
For the LASEK technique, a 20% alcohol solution (diluted in BSS) was instilled inside an 8-mm corneal marker centered on the pupil and left for 40s. The ablation was performed with the Esiris Schwind excimer laser using a PRK nomogram. The optical zone was determined by the mesopic pupil size. When the ablation depth exceeded 50μm, a 7-mm round cellulose sponge soaked in MMC 0.02% was applied for 30s over the ablated stroma, carefully avoiding leakage of the drug to the epithelial flap and the limbus.14 Because no study has demonstrated yet the exact ablation depth below which there is no risk of haze, we arbitrarily set the cut-off for using prophylactic MMC at 50μm of ablation depth, as previously reported.15 In these cases, because of the use of MMC, the programmed ablation was 10% less than the intended correction to avoid overcorrection. In the cases in which the ablation depth was 50μm or less, no MMC was applied. The stroma then was rinsed copiously with BSS, and the epithelial flap was repositioned using the same cannula. A therapeutic soft contact lens (Balafilicon A; Bausch & Lomb, USA) was placed carefully on the eye and the same drops as in FSBK were applied.
Postoperative follow-upThe medications consisted of topical antibiotic (ciprofloxacine 3mg/mL; Oftacilox; Alcon Cusí) and steroid (dexamethasone alcohol 1mg/mL; Maxidex; Alcon Cusí) drops 4 times daily after both procedures during the first week postoperatively. In patients who underwent FSBK, the drops were stopped after the first week; only artificial tears were continued thereafter. In patients who underwent LASEK, steroid drops were tapered over the subsequent two months: three times daily the first month, twice daily for the following 15 days and once daily for another 15 days. The therapeutic contact lens was removed 1 week after surgery.
Examinations were scheduled at 1 day, 1 week, 3 and 6 months postoperatively. Two optometrists, masked for the preoperative refraction and type of surgery, did the refractions at each postoperative visit. All the patients were refracted at the same room with the same light adjusted to mesopic conditions. Three and six months after surgery, a complete ocular examination including corneal topography (CSO; Compagnia Strumenti Oftalmici, Firenze, Italy) was performed.
Statistical analysisThe Statview SE+Graphics software (Abacus Concept Inc, Cupertino, CA, USA) was used for data analysis. Statistical comparisons were made using the unpaired two-tailed Student t test or Chi-square test when appropiate. P≤0.05 was considered statistically significant. The visual acuity (VA) was obtained in decimal scale, and was converted to logarithm of the minimal angle of resolution (logMAR) for statistical analysis using a VA conversion chart. All continuous data are expressed as the mean±the standard deviation.
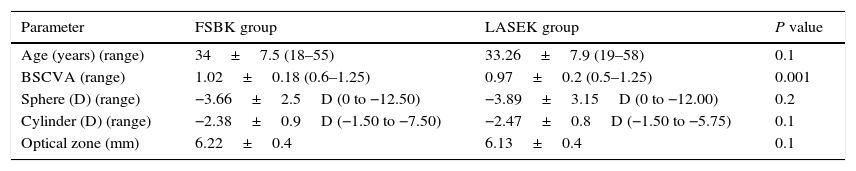
ResultsA total of 852 eyes were analyzed (427 FSBK and 425 LASEK eyes). In the LASEK group, 379 eyes (89.1%) received intraoperative MMC. Preoperative data are shown in Table 1. The preoperative BSCVA was significantly higher in the FSBK group (1.02±0.18) than in the LASEK group (0.97±0.20) (P=0.001). No statistically significant differences were found in the preoperative spherical and cylindrical defects between groups.
Preoperative refractive data of 427 eyes treated with FSBK and 425 eyes treated with LASEK for the correction of moderate to high astigmatism.
| Parameter | FSBK group | LASEK group | P value |
|---|---|---|---|
| Age (years) (range) | 34±7.5 (18–55) | 33.26±7.9 (19–58) | 0.1 |
| BSCVA (range) | 1.02±0.18 (0.6–1.25) | 0.97±0.2 (0.5–1.25) | 0.001 |
| Sphere (D) (range) | −3.66±2.5D (0 to −12.50) | −3.89±3.15D (0 to −12.00) | 0.2 |
| Cylinder (D) (range) | −2.38±0.9D (−1.50 to −7.50) | −2.47±0.8D (−1.50 to −5.75) | 0.1 |
| Optical zone (mm) | 6.22±0.4 | 6.13±0.4 | 0.1 |
BSCVA, best spectacle-corrected visual acuity; D, Diopters; FSBK, femtosecond laser sub-Bowman keratomileusis; LASEK,laser-assisted sub-epithelial keratomileusis.
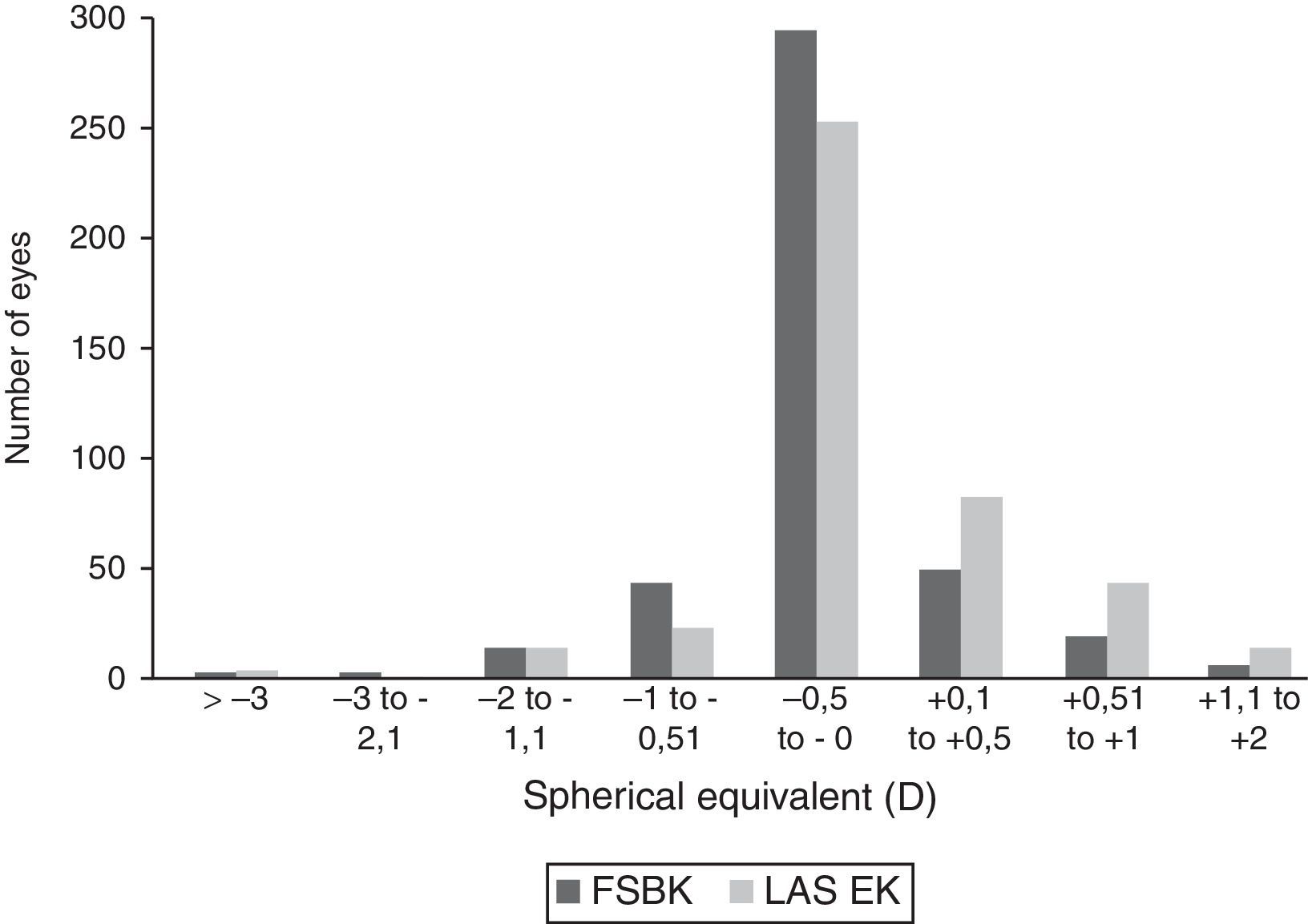
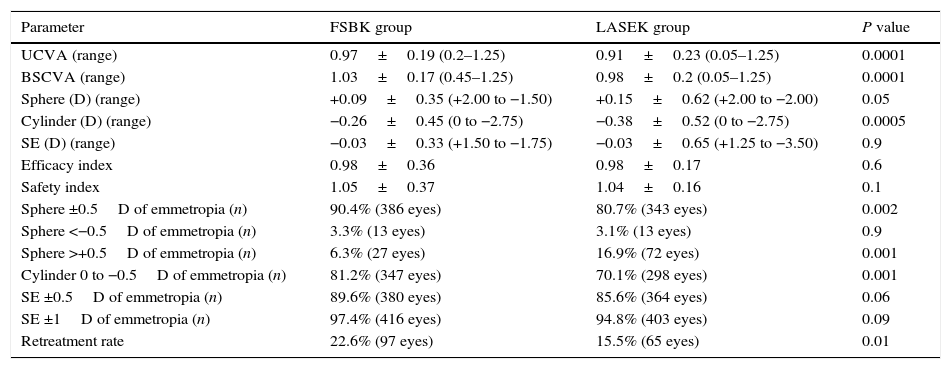
Table 2 shows the visual and refractive results 6 months after surgery in both groups. Six months postoperatively, the residual spherical defect was slightly but significantly higher in the LASEK group (+0.15±0.62D) than in the FSBK group (+0.09±0.35D) (P=0.05). The postoperative residual astigmatism was also slightly but significantly higher in the LASEK group (−0.38±0.52D) than in the FSBK group (−0.26±0.45D) (P=0.0005). The residual spherical equivalent (SE) was similar in both groups: FSBK −0.03±0.33D and LASEK −0.03±0.65D (P=0.9).
Six-months postoperative refractive data of 427 eyes treated with FSBK and 425 eyes treated with LASEK for the correction of moderate to high astigmatism.
| Parameter | FSBK group | LASEK group | P value |
|---|---|---|---|
| UCVA (range) | 0.97±0.19 (0.2–1.25) | 0.91±0.23 (0.05–1.25) | 0.0001 |
| BSCVA (range) | 1.03±0.17 (0.45–1.25) | 0.98±0.2 (0.05–1.25) | 0.0001 |
| Sphere (D) (range) | +0.09±0.35 (+2.00 to −1.50) | +0.15±0.62 (+2.00 to −2.00) | 0.05 |
| Cylinder (D) (range) | −0.26±0.45 (0 to −2.75) | −0.38±0.52 (0 to −2.75) | 0.0005 |
| SE (D) (range) | −0.03±0.33 (+1.50 to −1.75) | −0.03±0.65 (+1.25 to −3.50) | 0.9 |
| Efficacy index | 0.98±0.36 | 0.98±0.17 | 0.6 |
| Safety index | 1.05±0.37 | 1.04±0.16 | 0.1 |
| Sphere ±0.5D of emmetropia (n) | 90.4% (386 eyes) | 80.7% (343 eyes) | 0.002 |
| Sphere <−0.5D of emmetropia (n) | 3.3% (13 eyes) | 3.1% (13 eyes) | 0.9 |
| Sphere >+0.5D of emmetropia (n) | 6.3% (27 eyes) | 16.9% (72 eyes) | 0.001 |
| Cylinder 0 to −0.5D of emmetropia (n) | 81.2% (347 eyes) | 70.1% (298 eyes) | 0.001 |
| SE ±0.5D of emmetropia (n) | 89.6% (380 eyes) | 85.6% (364 eyes) | 0.06 |
| SE ±1D of emmetropia (n) | 97.4% (416 eyes) | 94.8% (403 eyes) | 0.09 |
| Retreatment rate | 22.6% (97 eyes) | 15.5% (65 eyes) | 0.01 |
UCVA, uncorrected visual acuity; BSCVA, best spectacle-corrected visual acuity; D, diopters; SE, spherical equivalent; FSBK, femtosecond laser sub-Bowman keratomileusis; LASEK, laser-assisted sub-epithelial keratomileusis.
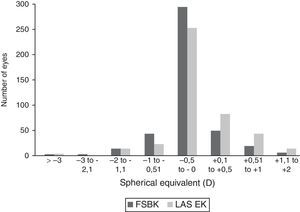
Six months after the surgery, the postoperative UCVA was significantly better (P=0.0001) in eyes treated with FSBK (0.97±0.19) than in eyes treated with LASEK (0.91±0.23). The postoperative BSCVA was significantly better in the FSBK group (1.03±0.17 versus 0.98±0.2 in the FSBK and LASEK groups, respectively) (P=0.0001). No significant differences were found in the efficacy and safety indexes between FSBK and LASEK (Table 2). The predictability of both procedures is shown in Fig. 1.
Comparison between femtosecond laser-assisted sub-Bowman keratomileusis (FSBK) and laser-assisted subepithelial keratomileusis (LASEK) spherical equivalent refractive outcomes 6 months after surgery. 89.9% of eyes in the FSBK group versus 85.6% of eyes in the LASEK group were within ±0.5D of emmetropia.
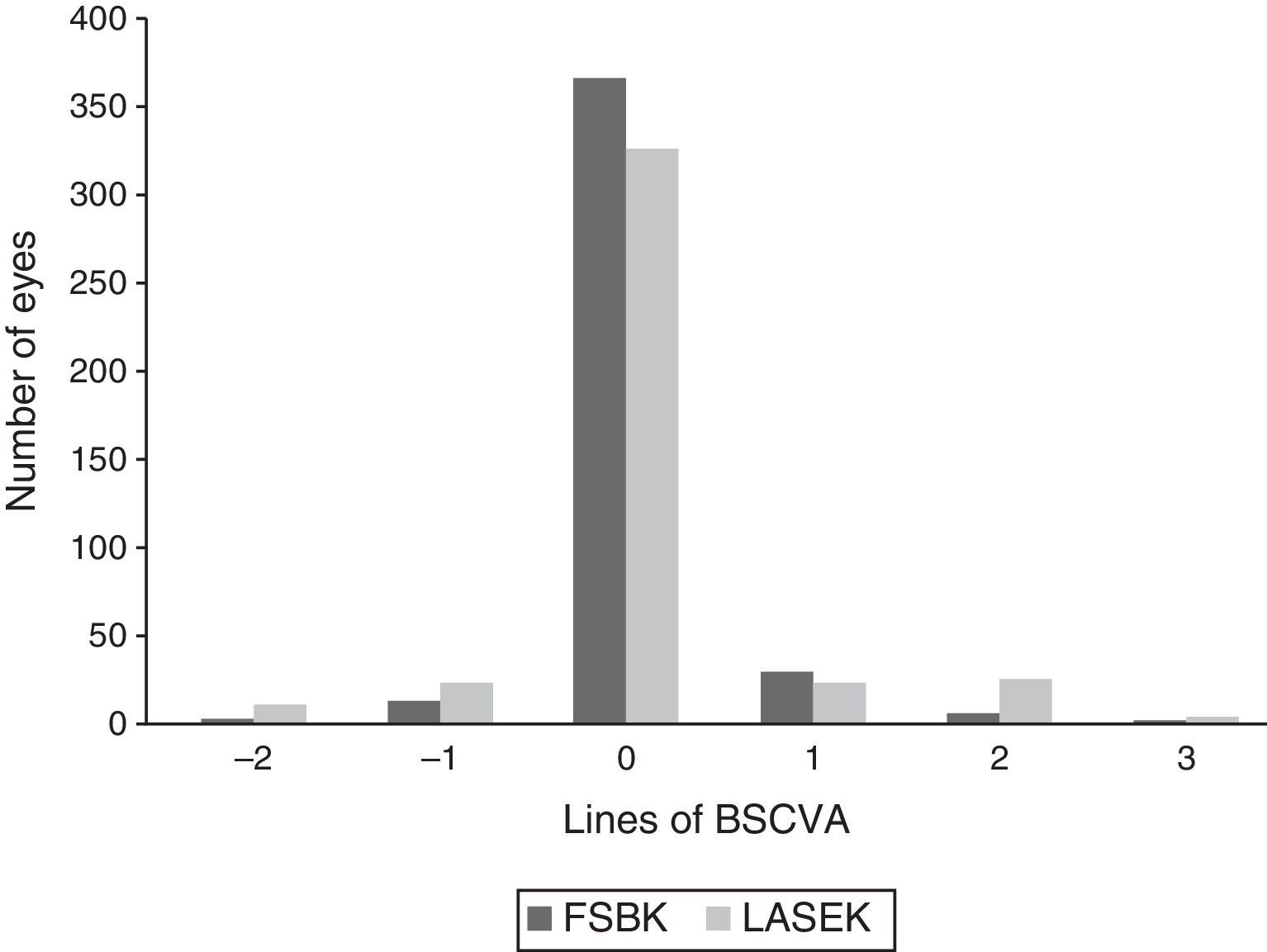
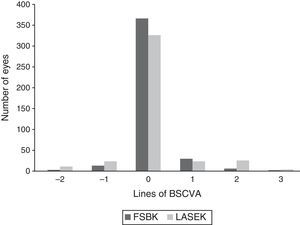
Changes in BSCVA 6 months after surgery are shown in Fig. 2. 16 eyes (3.7%) in the FSBK group versus 34 eyes (8.0%) in the LASEK group lost 1 or more lines of BSCVA. 37 eyes (8.6%) in the FSBK group gained 1 or more lines of BSCVA, whereas 52 eyes (12.1%) in the LASEK group gained 1 or more lines. Six months postoperatively, the enhancement rate was significantly higher in the FSBK group (22.6%) than in the LASEK group (15.5%) (P=0.01).
Regarding the complications, in the FSBK group, one patient experienced stage 2 diffuse lamellar keratitis, that required flap lift and stromal wash with BSS. 3 months after, the UCVA was 1.0. Another patient developed epithelial ingrowth that required flap lift and intraoperative application of MMC 0.02% for one minute. 3 months after, the UCVA was 0.85 with a residual refraction of +0.50, −1.00×170° and a BSCVA of 1.0. In the LASEK group, one patient developed clinically significant haze (grade III in subjective slit-lamp evaluation16) in the left eye one month after enhancement. MMC was applied for 2min over the stroma, resulting in a final UCVA of 0.4 and a BSCVA of 0.76 with a residual refraction of +2.00, −0.50×120°.
DiscussionOur results show that both FSBK and LASEK were safe and effective procedures to correct moderate to high myopic astigmatism (≥−1.50D). We found statistically significantly better visual and refractive results after FSBK 6 months after surgery. Although the differences were very small and probably not clinically relevant, regarding the highly demanding requirements of these procedures, the slightest difference between surgical techniques should be pointed out.
Compared to sphere, the treatment of high astigmatism requires the alignment of the elliptic ablation axis and possibly the compensation for both the ocular cyclotorsion and the “coupling” effect of the toric ablation on the spherical component.17
For these reasons, the treatment of high astigmatism seems to be less predictable than the correction of myopia regardless the selected ablation profile. In fact, in moderate to high astigmatism and using a LASIK technique, Ivarsen et al.3 described a significant undercorrection of the spherical equivalent (SE) (87% of patients were within ±0.5D of emmetropia) with FemtoLASIK and a MEL80 flying spot laser using an aspheric algorithm; Arbelaez et al.18 described that 72% of their patients were within ±0.5D of emmetropia (SE) with FemtoLASIK and an aberration-free ablation profile centered in corneal apex; Katz et al.13 found that 67% of patients were within ±0.5D of emmetropia (SE) using microkeratome and a wavefront-optimized excimer ablation; Payvar et al.19 obtained an even lower 33% of patients with a SE within ±0.5D of emmetropia using the Nidek EC 5000 laser, although these poor results were improved by others20,21 using a different ablation nomogram with the same laser. In the current study, we found that 89.6% of FSBK-treated eyes had an SE within ±0.5D of emmetropia.
On the other hand, regarding SA procedures for the correction of moderate to high astigmatism, Fring et al.22 found that 76% of patients had an SE within ±0.5D of emmetropia using LASEK with MMC and the MEL80 excimer laser; Hamberg-Nystrom et al.23 using photoastigmatic keratectomy found that 80% of patients were within ±1D of SE in high astigmatism; Sedhipur et al.24 using PRK with MMC found that 72% and 90% of patients had a SE within ±1D of emmetropia depending on the laser algorithm applied (cross cylinder versus single method). In the current study, 85.6% of LASEK-treated eyes had an SE within ±0.5D of emmetropia.
However, and to the best of our knowledge, only one study, performed by Katz et al.,13 has been specifically designed to compare the visual and refractive results of LASIK (performed with a mechanical microkeratome) versus SA for the correction of high astigmatism (using in both groups a wavefront-optimized ablation algorithm, unlike our study). The authors found no significant differences in the visual and refractive results after mechanical LASIK compared to PRK. However, the low number of patients included in the study (57 eyes in each group) may have limited the possibility of finding small differences between the two groups. In our study, with a much larger population, we found a slightly better UCVA and better refractive results favoring FSBK compared to LASEK at the 6-month postoperative examination. We performed our analysis six months after surgery as it is known that VA tends to improve after SA during this period.25 In addition, the efficacy indexes obtained in our study (0.98±0.36 after FSBK and 0.98±0.17 after LASEK) are higher than those obtained by Katz et al.13 (0.74±0.19 in the mechanical LASIK group and 0.76±0.32 in the PRK group). Several hypothesis might explain these differences. First, Katz and colleagues13 used a mechanical microkeratome for the LASIK flap-creation whereas we used a femtosecond laser. It is well accepted that the femtosecond laser creates a predictably thin and planar corneal flap10,11 that is thought to have less biomechanical impact on the cornea than the thicker, meniscus-shaped flap obtained with a mechanical microkeratome.11 In fact, femtosecond laser seems to induce less higher-order aberrations26 and less changes in corneal curvature27 compared to mechanical microkeratome. In addition, the flap diameter obtained with a mechanical microkeratome is conditioned by the corneal curvature (i.e., corneas with high curvature predispose to obtain a flap diameter higher than the intended),28 whereas the femtosecond laser-created flap diameter tends to be much predictable,29 regardless of the corneal curvature. Second, the mean preoperative cylindrical defect was higher in the study performed by Katz et al.13 which might explain the poorer efficacy. And third, as a multicenter study with 19 surgeons,13 the variability of the refractive results would be higher than ours, where only one center and two surgeons were involved.
On the other hand, we want to remark the fact that, although the efficacy indexes obtained in our study are quite high (0.98±0.36 after FSBK and 0.98±0.17 after LASEK), the retreatment rates we found are also high (22.6% in the FSBK group and 15.5% in the LASEK group). Therefore, it could be hypothesized that the efficacy index alone is not sensitive enough to detect residual refractive errors, at least when analyzing treatment results of high myopic astigmatism. In addition, it is noteworthy the discrepancy found between the low mean residual SE (−0.03±0.33D in the FSBK group and −0.03±0.65D in the LASEK group) and the high enhancement rates of both techniques. For this reason, we believe that residual sphere and cylinder must be analyzed separately, at least when treatment of high myopic astigmatism is evaluated. Regarding the residual spherical refraction, both techniques showed a slight tendency toward overcorrection 6 months after the surgery (+0.09D in the FSBK group and +0.15D in the LASEK group) (P=0.05). As previously published by other authors,25,30 the presence of hyperopic shift after SA is common; it might be attributed to the corneal wound-healing response and tends to improve and stabilize six months after the surgery.25 On the other hand, regarding the astigmatism, we found a low mean residual cylindrical defect after both procedures (−0.26±0.45D in the FSBK group and −0.38±0.52D in the LASEK group) (P=0.0005). Our results support the findings of previous studies that suggest the lower predictability of LASIK3,18 and LASEK22 when treating high astigmatism as compared to the results obtained when treating unselected myopic patients.31 Moreover, by performing a single-center comparative study and including a high number of patients, the current study avoids some well-known factors that may biass the results (i.e., different laser platform, different ablation algorithm, different ablation zone, different MMC dosage, etc.).
It is well known that vectorial analysis is the only way to evaluate the surgically induced astigmatism (SIA), and thus to find the degree of “matching” of this parameter with the target correction programmed. With this kind of analysis, it is possible to make refinements of the ablation algorithm because the investigator is able to calculate the degree of hyper or hypo correction of the astigmatism, and also the degree of axis mismatch between the intended and the actually obtained correction. Nevertheless, as the main goal of our study was just to describe the 6-month postoperative visual and refractive results after FSBK and LASEK when treating moderate to high myopic astigmatism, the vectorial analysis is not needed.
It is also important to note that patients with high myopic astigmatism should be advised of an expected higher retreatment rate as compared to the laser correction of pure myopia.32 Furthermore, the higher retreatment rate found with FSBK compared to LASEK in our study (22.6% versus 15.5%) might be explained by the faster visual recovery and less postoperative discomfort after FSBK as compared to SA that may make patients more prone for an enhancement in the FSBK group.
On the other hand, and given the fact that no clinically significant differences in the visual and refractive results were detected in the current study between FSBK and LASEK when treating moderate to high astigmatism, the surgical technique of choice should depend on other parameters, such as thin central corneal thickness, topographic features, and patient needs or preferences.
ConclusionIn conclusion, we found that both FSBK and LASEK are safe and effective procedures to correct moderate to high myopic astigmatism. Visual recovery is slower after LASEK, and slightly better visual and refractive results seem to be obtained with FSBK in a 6-month follow-up. More studies with longer follow-up and studies dealing with the biomechanical response of the cornea are needed to further improve our knowledge about high astigmatism laser correction.
Conflicts of interestThe authors have no conflicts of interest to declare. No author has a financial or proprietary interest in any materials or methods mentioned.