The aim of this study was to evaluate the effects of pharmacologic mydriasis and Peripheral Iridotomy (PI) on ocular biometry and anterior chamber parameters in primary angle closure suspects.
MethodsIn this prospective interventional case series, 21 primary angle closure suspects were enrolled. Intraocular pressure, refraction, ocular biometry (Lenstar, LS900), and anterior chamber parameters (Pentacam HR) were measured at four occasions: before PI (before and after mydriasis with phenylephrine) and two weeks after PI (before and after mydriasis). The study was conducted on both eyes and only one eye per patient, in random, was included in the analysis.
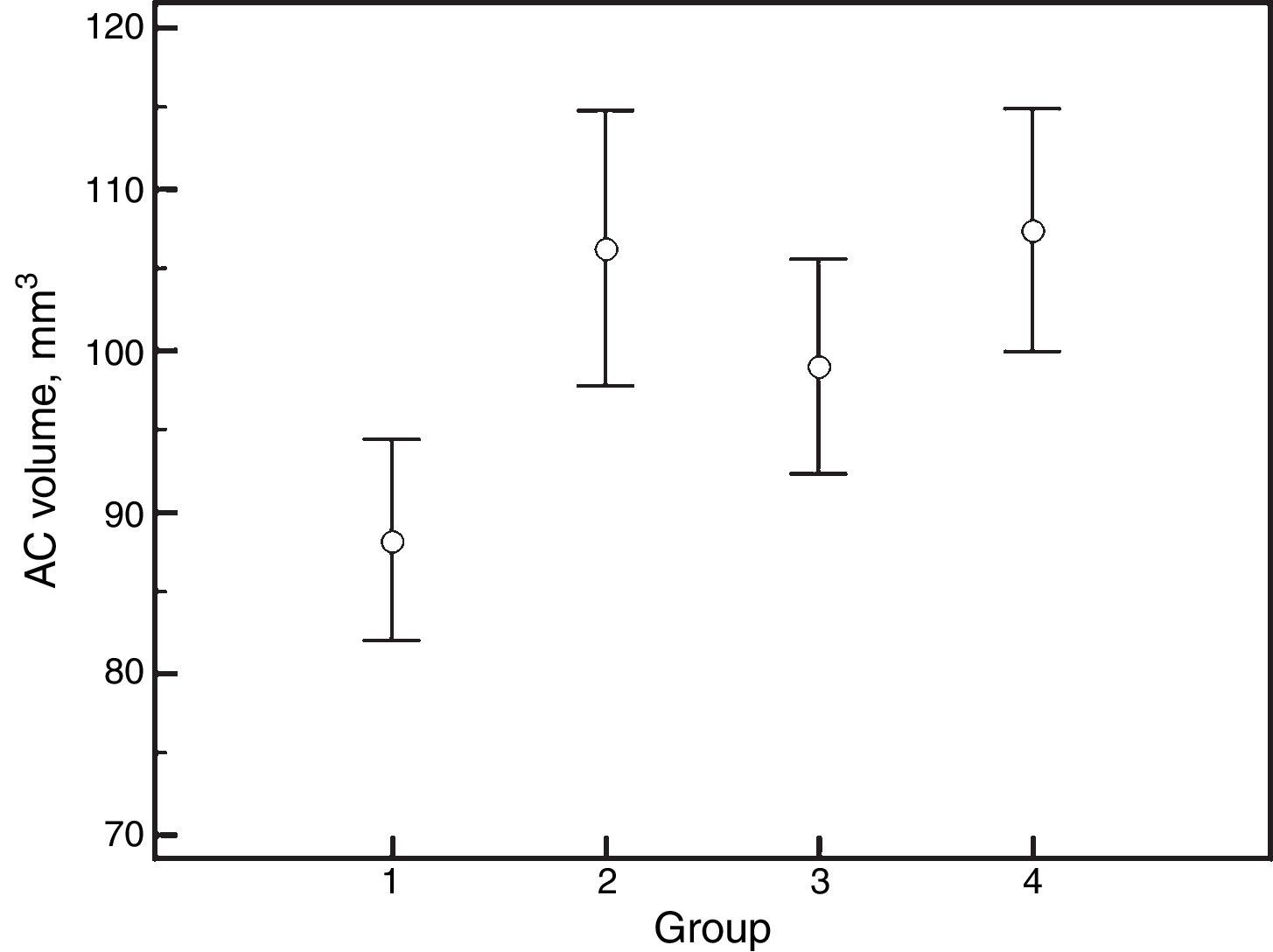
ResultsThe mean age of the participants was 60±7 years and 17 (81%) were female. There were no significant differences in intraocular pressure, refraction, keratometry, biometric and anterior chamber parameters between groups, except for anterior chamber volume, which showed increments with PI and mydriasis. The corresponding values for anterior chamber volume were as follows: 88.2±13.7mm3 before PI, undilated; 106.3±18.8 before PI, dilated; 99.0±14.6 after PI, undilated, and 107.4±16.5 after PI, dilated (P<0.001).
ConclusionsThis study showed no change in the ocular biometric and anterior chamber parameters including iridocorneal angle after PI and/or pharmacologic mydriasis except for increments in anterior chamber volume. This factor has the potential to be used as a numerical proxy for iris position in evaluating and monitoring patients with primary angle closure suspects after PI.
El objetivo de este estudio fue el de evaluar los efectos de la midriasis farmacológica y la iridotomía periférica (IP) en la biometría ocular y los parámetros de la cámara anterior en las sospechas de cierre angular primario.
MétodosEn esta serie de casos intervencional prospectiva, se incluyó a 21 sospechas de cierre angular primario. Se realizaron las mediciones siguientes: presión intraocular, refracción, biometría ocular (Lenstar, LS900), y parámetros de la cámara anterior (Pentacam HR) en cuatro ocasiones, antes de la IP (antes y después de la midriasis con fenilefrina) y dos semanas después de la IP (antes y después de la midriasis). El estudio se realizó en ambos ojos, incluyéndose en el análisis un solo ojo por paciente de manera aleatoria.
ResultadosLa edad media de los participantes fue de 60±7 años, de los cuales 17 eran mujeres (81%). No se hallaron diferencias significativas en cuanto a presión intraocular, refracción, queratometría, parámetros biométricos y de la cámara anterior entre los grupos, exceptuando el volumen de la cámara anterior, que reflejó incrementos con la IP y la midriasis. Los valores correspondientes para el volumen de la cámara anterior fueron los siguientes: 88.2±13,7mm3 antes de la IP, sin dilatación; 106.3±18,8 antes de la IP, con dilatación; 99.0±14,6 tras la IP, sin dilatación, y 107.4±16,5 tras la IP, con dilatación (P<0,001).
ConclusionesEl presente estudio no reflejó cambios en los parámetros biométricos oculares y de la cámara anterior, incluyendo el ángulo iridocorneal tras la IP y/o midriasis farmacológica, exceptuando los incrementos del volumen de la cámara anterior. Este factor tiene el potencial de ser utilizado como indicador numérico de la posición del iris al evaluar y supervisar a los pacientes con sospechas de cierre angular primario tras IP.
Primary angle-closure glaucoma (PACG) is believed to be one of the leading causes of irreversible blindness worldwide, and it is estimated that 26% of 80 million glaucomatous patients by 2020 will have PACG.1 It was reported that approximately 10% of people who have anatomically narrow angles (Primary Angle Closure Suspects, PACS) eventually develop angle closure glaucoma.2 Factors that convert a PACS patient to acute primary angle-closure (APAC) or chronic angle-closure glaucoma (CACG) are unknown, and it would be of interest to know why certain eyes get involved with acute attack, some others with CACG, and some develop no sign of glaucoma. Therefore, any study about the anterior chamber (AC) parameters and ocular biometry may ultimately help us to learn more about the pathophysiology of glaucoma development in PACS.
The rationale for this study stems from the knowledge that in instances of increased ocular sympathetic tone, including emotional distress, low light conditions, or after sympathomimetic drug use, the iris dilator muscles contract and lead to pupil dilatation. The pupil dilation may result in pupillary block and development of APAC.3 Moreover, it has been shown that AC parameters could be changed after pharmacologic mydriasis.4 We aimed to measure any change in the ocular biometric characteristic and AC parameters using Lenstar LS 900 biometer (Haag-Streit AG, Koeniz, Switzerland) and Pentacam HR (Oculus, Wetzlar, Germany) before and after pharmacologic mydriasis in patient with PACS before and after Peripheral Iridotomy (PI).
Both Lenstar and Pentacam HR are among recently developed technologies that can objectively measure various AC parameters. Lenstar uses a partial coherence interferometer, and can measure several biometric parameters including central corneal thickness (CCT), central AC depth, lens thickness (LT), and axial length (AL).5,6 Pentacam HR incorporates a rotating Scheimpflug camera that captures several cross-sectional images to evaluate the whole anterior segment of the eye from the surface of the cornea to the posterior surface of the lens. It uses height information from up to 25,000 data points to calculate parameters such as CCT, corneal curvature, AC angle, volume, and depth.7 Both devices offer objective and reproducible information for AC biometry.8,9
Several previous studies have evaluated the effect of PI on the AC and angle parameters using different technologies such as optical coherence tomography, ultrasound biomicroscopy, partial coherence interferometry, and Scheimpflug imaging.10–16 However, the effect of PI on some AC parameters remains controversial and in none of them the effect of pharmacologic mydriasis has been evaluated before and after PI.10,13,14
Materials and methodsStudy populationIn this prospective interventional study, refraction, intraocular pressure (IOP), and biometric data from Lenstar and Pentacam HR were collected from 21 consecutive patients with PACS among the patients referred for glaucoma evaluation to the glaucoma service of a tertiary care centre. PACS was defined as at least 270° iridotrabecular apposition without synechial changes, normal IOP, and normal optic disc features. Cases with positive history (or objective signs) of ocular disorders other than PACS (e.g., glaucoma, uveitis, corneal ectatic disorders, and diabetic retinopathy), previous ocular surgery or trauma, hypertension, topical or systemic anticholenergic or sympathomimetic agents use, or inability to cooperate with any measurement device were excluded. Detailed informed consent was obtained from all participants before enrolment. The study adhered to the tenets of the Declaration of Helsinki, and the research protocol was approved by the Ethics Committee at Shiraz University of Medical Sciences.
Procedures and measurementsAll patients who met the inclusion criteria underwent a complete ocular examination including visual acuity measurement, tonometry, slit-lamp biomicroscopy, and fundus exam using a 90-diopter noncontact lens. Then IOP, refraction, ocular biometric parameters (using Lenstar LS 900), and AC characteristics (using Pentacam HR) were measured before and after mydriasis. All patients underwent PI a week later. Two weeks after PI, all biometric and AC parameters, IOP, and refraction were measured before and after pharmacologic mydriasis. In order to eliminate any possible effect of cycloplegic agents on the ciliary muscles and lens position, mydriasis was achieved with phenylephrine 5% drops, applied every 10min for a total of 4 times. After the first set of post-mydriasis measurements (before PI), all patients were observed for 3h after dilation and were informed about symptoms of acute IOP elevation, They also were advised to refer to our ophthalmology emergency room if any of the discussed symptoms developed. None of the patients experienced acute IOP rise.
Refractions were measured by an autorefractometer (KR-8800 Kerato-Refractometer, Topcon Inc., Japan), and IOPs were recorded from a non-contact computerized tonometer (CT.80, Topcon Inc., Japan). The following parameters were collected from Lenstar: Mean Keratometry (Km), Keratometric Astigmatism (Ka), Central Corneal Thickness (CCT), axial length (AL), central AC depth (from endothelium to anterior lens surface), and Lens Thickness (LT). Pentacam HR was used to obtain Km, central AC depth (from endothelium to anterior lens surface), AC volume, and AC angles at superior, temporal, inferior, and nasal quadrants. The following factors were calculated from Lenstar recordings: Lens-AL factor [LAF=(LT/AL)×10]; lens position (LP=AC depth+0.5×LT); and relative lens position [RLP=(LP/AL)×10]. Each instrument was calibrated at the beginning of the study, and at regular intervals afterwards (as per manufacturer recommendations). All measurements from each device were performed under standard dim-light conditions by the same experienced investigator using criteria provided by the manufacturers of devices.
Peripheral iridotomyPI was performed for each eye using Abraham iridotomy lens (Volk Optical Inc., OH), and a commercial ophthalmic Nd:YAG laser system (Nidek YC-1800, Nidek Inc., Japan). The energy was initially set at 5mJ/pulse, and about 3–5 shots were applied to achieve a full thickness hole at 12:00 o’clock position by the same glaucoma specialist (M.R.R.). Postoperative systemic (oral acetazolamide, 500mg stat) and topical (betamethasone, 4 times a day for a maximum of 10 days) medications were prescribed. The patency of the PIs were assessed and confirmed by retro-illumination.
Statistical analysisData were recorded and analyzed using IBM SPSS Statistics software version 21 (SPSS Inc., Chicago, IL) and MedCalc version 12.2.1 (MedCalc Software, Mariakerke, Belgium). Only one eye per patient, in random, was included in the analysis. Descriptive statistics were presented as mean±standard deviation (SD). Comparisons between the 4 groups were performed with repeated-measures ANOVA test. A P value of less than 0.05 was considered as statistically significant.
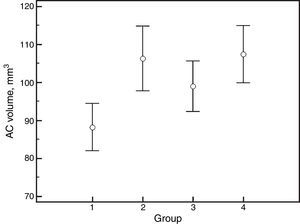
ResultsThe mean age of the participants was 60±7 years (median, 60; range, 47–74) and 17 out of 21 (81%) were female. There were no significant differences between groups for IOP, refraction, keratometry, ocular biometry and AC parameters, except for AC volume, which showed increments with PI or mydriasis (Tables 1–3). The corresponding values for AC volume were as follows: 88.2±13.7mm3 (before PI and non-mydriatic); 106.3±18.8mm3 (before PI and mydriatic); 99.0±14.6mm3 (after PI and non-mydriatic); and 107.4±16.5mm3 (after PI and mydriatic) (P<0.001; Fig. 1). Pair-wise comparisons revealed that mydriasis significantly increased the AC volume both before and after PI; the PI in non-mydriatic eye was significantly associated with increased AC volume, while such association was not observed in mydriatic eyes. In non-mydriatic eyes, the mean difference in AC volume for pre- vs. post-PI measurements was 10.8±6.5 mm3, which is 12% of the baseline volume.
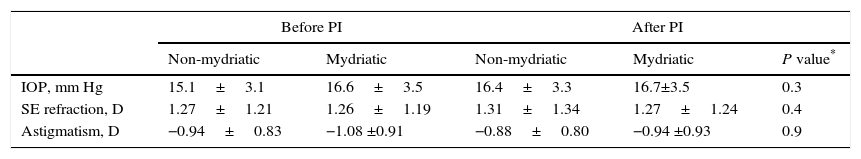
Changes in intraocular pressure and refraction after pharmacologic mydriasis and/or peripheral iridotomy in primary angle closure suspects.
| Before PI | After PI | ||||
|---|---|---|---|---|---|
| Non-mydriatic | Mydriatic | Non-mydriatic | Mydriatic | P value* | |
| IOP, mm Hg | 15.1±3.1 | 16.6±3.5 | 16.4±3.3 | 16.7±3.5 | 0.3 |
| SE refraction, D | 1.27±1.21 | 1.26±1.19 | 1.31±1.34 | 1.27±1.24 | 0.4 |
| Astigmatism, D | −0.94±0.83 | −1.08 ±0.91 | −0.88±0.80 | −0.94 ±0.93 | 0.9 |
D, diopter; IOP, intraocular pressure; PI, peripheral iridotomy; SE, spherical equivalent.
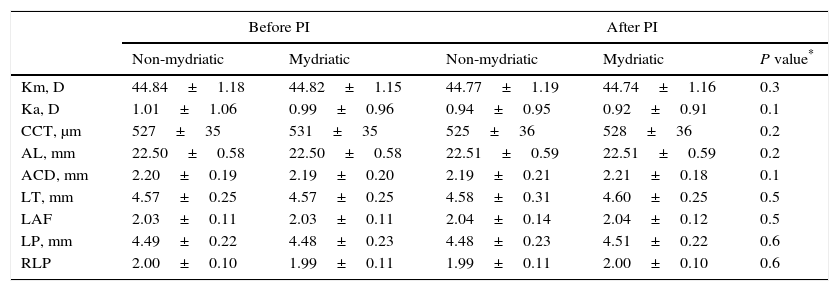
Changes in IOL master keratometric and biometric factors after pharmacologic mydriasis and/or peripheral iridotomy in eyes with primary angle closure suspects.
| Before PI | After PI | ||||
|---|---|---|---|---|---|
| Non-mydriatic | Mydriatic | Non-mydriatic | Mydriatic | P value* | |
| Km, D | 44.84±1.18 | 44.82±1.15 | 44.77±1.19 | 44.74±1.16 | 0.3 |
| Ka, D | 1.01±1.06 | 0.99±0.96 | 0.94±0.95 | 0.92±0.91 | 0.1 |
| CCT, μm | 527±35 | 531±35 | 525±36 | 528±36 | 0.2 |
| AL, mm | 22.50±0.58 | 22.50±0.58 | 22.51±0.59 | 22.51±0.59 | 0.2 |
| ACD, mm | 2.20±0.19 | 2.19±0.20 | 2.19±0.21 | 2.21±0.18 | 0.1 |
| LT, mm | 4.57±0.25 | 4.57±0.25 | 4.58±0.31 | 4.60±0.25 | 0.5 |
| LAF | 2.03±0.11 | 2.03±0.11 | 2.04±0.14 | 2.04±0.12 | 0.5 |
| LP, mm | 4.49±0.22 | 4.48±0.23 | 4.48±0.23 | 4.51±0.22 | 0.6 |
| RLP | 2.00±0.10 | 1.99±0.11 | 1.99±0.11 | 2.00±0.10 | 0.6 |
ACD, anterior chamber depth; AL, axial length; CCT, central corneal thickness; D, diopter; IOP, intraocular pressure; Ka, keratometric astigmatism; Km, mean keratometry; LAF, lens axial length factor; PI, peripheral iridotomy; LT, lens thickness; LP, lens position; RLP, relative lens position.
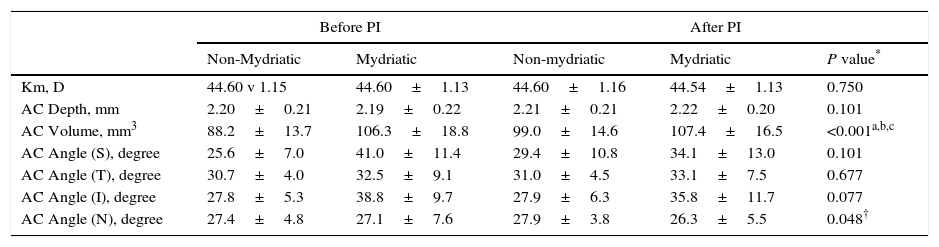
Changes in Pentacam HR keratometric and biometric parameters after pharmacologic mydriasis and/or peripheral iridotomy in primary angle closure suspects.
| Before PI | After PI | ||||
|---|---|---|---|---|---|
| Non-Mydriatic | Mydriatic | Non-mydriatic | Mydriatic | P value* | |
| Km, D | 44.60 v 1.15 | 44.60±1.13 | 44.60±1.16 | 44.54±1.13 | 0.750 |
| AC Depth, mm | 2.20±0.21 | 2.19±0.22 | 2.21±0.21 | 2.22±0.20 | 0.101 |
| AC Volume, mm3 | 88.2±13.7 | 106.3±18.8 | 99.0±14.6 | 107.4±16.5 | <0.001a,b,c |
| AC Angle (S), degree | 25.6±7.0 | 41.0±11.4 | 29.4±10.8 | 34.1±13.0 | 0.101 |
| AC Angle (T), degree | 30.7±4.0 | 32.5±9.1 | 31.0±4.5 | 33.1±7.5 | 0.677 |
| AC Angle (I), degree | 27.8±5.3 | 38.8±9.7 | 27.9±6.3 | 35.8±11.7 | 0.077 |
| AC Angle (N), degree | 27.4±4.8 | 27.1±7.6 | 27.9±3.8 | 26.3±5.5 | 0.048† |
Pairwise comparisons were carried out by LSD method, and pairs with significant P values were shown by superscript alphabets as follows: a: before PI – non-mydriatic vs. before PI – mydriatic; b: after PI – non-mydriatic vs. after PI – mydriatic; C: before PI – non-mydriatic vs. after PI – non-mydriatic; d: before PI – mydriatic vs. after PI – mydriatic.
AC, anterior chamber; D, diopter; I, inferior; IOP, intraocular pressure; Km, mean keratometry; PI, peripheral iridotomy; N, nasal; S, superior; T, temporal.
Although we did not find any statistically significant change in the AC angle after mydriasis and/or PI, a trend towards greater values after PI (in non-mydriatic eyes) or mydriasis was observed (Table 3). The mean of the AC angle at the 4 locations was 27.5±3.6° vs. 28.5±3.4° for non-mydriatic eyes before and after PI, respectively (P=0.093; paired-samples T-test). For mydriatic eyes, the greater fluctuation between the mean values of different locations and greater standard deviations may imply the worse accuracy of Pentacam HR for AC angle measurement in the mydriatic state (Table 3).
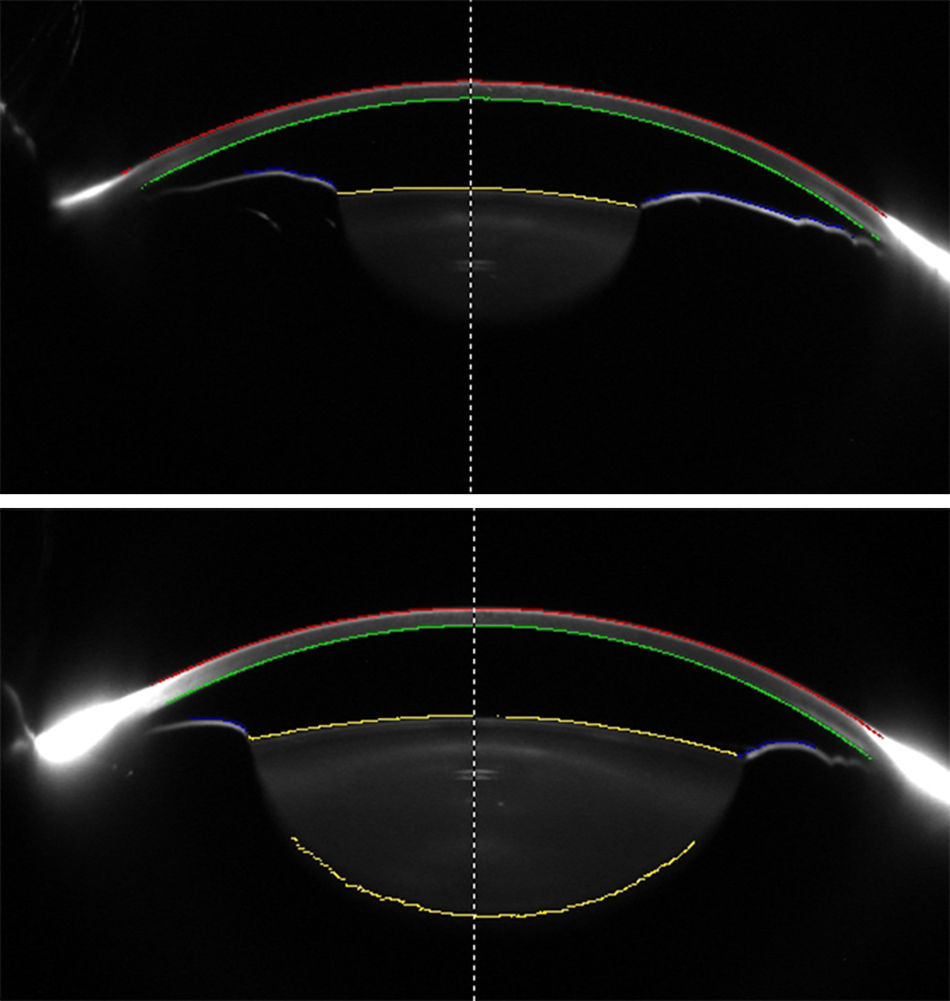
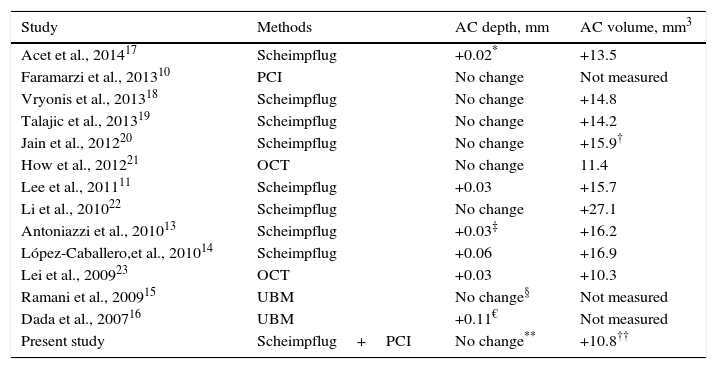
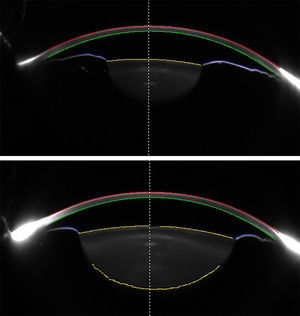
DiscussionThe results of the present study showed that PI had no significant effect on refraction, IOP, corneal curvature, and ocular biometric parameters in eyes with PACS, except for AC volume that significantly increased after PI and mydriasis. Previous studies were inconclusive regarding the effect of PI on AC depth (increased vs. no change) in early postoperative period; however, a majority of studies advocated the notion that central AC depth does not change after PI (Table 4). Using two precise devices with different technologies, our study further corroborates this notion. In addition, the differences in AC depth found in some studies (0.02–0.11mm) were not clinically relevant, and could be a product of normal physiologic changes (e.g., diurnal) or repeatability issues of devices. These concepts are also supported from the theoretical point of view: central AC depth is measured by calculating the distance from the corneal endothelium to the anterior lens surface (Fig. 2, top); PI primarily affects the iris contour and has no effect on the lens position, and hence is unlikely to affect AC depth measurements.
Effect of laser peripheral iridotomy on the early (within 1 month) postoperative measurements of AC depth and volume according to different studies.
| Study | Methods | AC depth, mm | AC volume, mm3 |
|---|---|---|---|
| Acet et al., 201417 | Scheimpflug | +0.02* | +13.5 |
| Faramarzi et al., 201310 | PCI | No change | Not measured |
| Vryonis et al., 201318 | Scheimpflug | No change | +14.8 |
| Talajic et al., 201319 | Scheimpflug | No change | +14.2 |
| Jain et al., 201220 | Scheimpflug | No change | +15.9† |
| How et al., 201221 | OCT | No change | 11.4 |
| Lee et al., 201111 | Scheimpflug | +0.03 | +15.7 |
| Li et al., 201022 | Scheimpflug | No change | +27.1 |
| Antoniazzi et al., 201013 | Scheimpflug | +0.03‡ | +16.2 |
| López-Caballero,et al., 201014 | Scheimpflug | +0.06 | +16.9 |
| Lei et al., 200923 | OCT | +0.03 | +10.3 |
| Ramani et al., 200915 | UBM | No change§ | Not measured |
| Dada et al., 200716 | UBM | +0.11€ | Not measured |
| Present study | Scheimpflug+PCI | No change** | +10.8†† |
AC, anterior chamber; OCT, optical coherence tomography; PCI, partial coherence interferometry; UBM, ultrasound biomicroscopy.
Scheimpflug images of an eye before (top) and after (bottom) pharmacologic mydriasis. Different surfaces of the anterior segment are traced by the device and used for analysis. Anterior Chamber (AC) depth is measured from the posterior cornea to the anterior lens surface at the midline (dashed line). Data from posterior cornea, anterior lens, and anterior iris surfaces are analyzed for calculating AC volume.
On the other hand, AC volume is calculated by tracing corneal endothelial, anterior lens, and anterior iris surfaces; thus, it is essentially influenced by the iris contour (Fig. 2). By flattening the iris curvature,24 PI has the potential to increase the calculated AC volume. Actually, almost all previous studies reported significant increase in AC volume after PI (Table 4), a finding that was also observed in our study. Therefore, AC volume may have the potential to be considered as a sensitive measure to evaluate changes in iris contour after PI. It may help to determine eyes that respond poorly to PI (with probably less AC volume change after PI), or monitor the efficacy of the PI through follow-up visits. However, AC volume is also determined by the AC depth and pupil size, and these two factors can confound measurements if left uncontrolled. In particular, the effect of pupil size on the AC volume was proved in our study, where pharmacologic mydriasis was shown to be associated with greater AC volume (compared to non-mydriatic status) in both pre- and post-PI eyes. Indeed, the positive effect of PI on AC volume was masked in mydriatic eyes, because the role of anterior iris surface in AC volume calculation is notably decreased when the iris is dilated. In other words, the pupil dilation did not result in any changes in the AC and ocular biometric parameters before and after PI except for the increment in AC volume that was due to measuring surface change from anterior iris surface to anterior lens capsule.
Although several specialized parameters have been developed to evaluate changes in iris contour and iridocorneal angle,16,24 the associated methods and devices are not in widespread use. Until then, the AC volume parameter of the more available Scheimpflug based devices may be used as a proxy for changes in iris contour, provided that the measurements are obtained in non-mydriatic state, and controlled for pupil size and AC depth (both of which are also provided by Scheimpflug technology).
In this study, we did not find any statistically significant change in AC angle parameters of the Pentacam HR. However, in non-mydriatic state, we found a trend towards greater AC angle values after performing PI (27.5° vs. 28.5°, respectively). This finding was in line with previous studies that used the same device and method for AC angle measurement.25,18,19,26,14 Among AC parameters measured by Pentacam HR, AC angle values showed the least reproducibility.27 This finding was attributed to the limitation of the Scheimpflug camera to obtain clear images from structure adjacent to limbus, where light scattering make a noise.25,18 Together, AC angle measurements of the Pentacam HR are not a very useful parameter to detect subtle alterations in the AC angle or iris contour.
The limitations of the study include being conducted in a single centre, and on a homogenous ethnic group, which limit its generalizability, and also lack of healthy controls. Additionally, the study only recruited subjects who had PACS, so our findings should not be extrapolated to those with PAC and PACG. However, it was a prospective study, and two precise devices with different technologies were used for evaluation of AC parameters, which expanded the measurable parameters and enhanced the overall validity of the results. Moreover, to the best of our knowledge, it was the first study that evaluated the effect of pure mydriasis (without cycloplegia) on AC parameters of patients with PACS using Pentacam HR.
In conclusion, according to the findings of the present study, AC volume was the only parameter that showed significant change after PI. This factor has the potential to be used as a numerical proxy for iris position in evaluating and monitoring patients with PACS. However, the confounding effect of mydriasis (and pupil size in general) should be taken into account.
Financial supportIranian Elite Foundation, Fars Branch.
Conflict of interestThe authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.