The purpose of this study was to evaluate the effect of pharmacologic pupillary dilatation on anterior chamber depth (ACD) and anterior chamber angle (ACA) in eyes with exfoliation syndrome (XFS).
MethodsThirty-six eyes of 36 patients with XFS were evaluated with slit-lamp examination, Goldmann applanation tonometry and ultrasound biomicroscopy (UBM) under standard light conditions. Primary outcome parameters were defined as the change in ACD and ACA measured before and 40min after instillation of a single drop of either 1% cyclopentolate (Group I; n=12), 2.5% phenylephrine (Group II; n=12) or 1% tropicamide (Group III; n=12). Change in intraocular pressure (IOP) during the same time interval was included as a secondary outcome measure.
ResultsThe average predilatation ACD, ACA and IOP values in the study subjects were 2.54±0.40mm, 27.9±6.3° and 14.9±3.1mmHg, respectively. There were no significant differences in the mean age (p=0.461), the female/male ratio (p=0.232), baseline ACD (p=0.841), ACA (p=0.761) or IOP (p=0.070) within the three groups. Differences in dilation induced changes in ACD (p=0.108), ACA (p=0.636) and IOP (p=0.160) between the three groups were not statistically significant.
ConclusionPupillary dilatation with a single drop of 1.0% cyclopentolate, 2.5% phenylephrine or 1% tropicamide is not associated with shallowing of the anterior chamber or narrowing of the ACA in patients with XFS who present with open angles.
El objetivo de este estudio fue el de evaluar el efecto de la dilatación farmacológica de la pupila sobre la profundidad de la cámara anterior (PCA) y el ángulo de la cámara anterior (ACA) en ojos con síndrome de exfoliación (SXF).
MétodosSe evaluaron treinta y seis ojos de 36 pacientes con SXF mediante una lámpara de hendidura, tonometría de aplanación de Goldmann y biomicroscopia ultrasónica (BMU), en condiciones de iluminación estándar. Se definieron como parámetros de medida primarios del resultado a los cambios de PCA y ACA medidos con anterioridad y a los 40 minutos de la instilación de una única gota de ciclopentolato al 1% (Grupo I; n=12), fenilefrina al 2,5% (Grupo II; n=12) o tropicamida al 1% (Grupo III; n=12). Se incluyó el cambio de la presión intraocular (PIO) durante el mismo intervalo de tiempo como medición secundaria del resultado.
ResultadosLos valores medios de PCA, ACA y PIO previos a la dilatación en los pacientes del estudio fueron de 2,54±0,40mm, 27,9±6,3° y 14,9±3,1mmHg, respectivamente. No existieron diferencias en cuanto a la edad media (p=0,461), el ratio hombre/mujer (p=0,232), PCA basal (p=0,841), ACA (p=0,761) o PIO (p=0,070) dentro de estos tres grupos. La diferencias en los cambios inducidos tras la dilatación en cuanto a PCA (p=0,108), ACA (p=0,636) y PIO (p=0,160) entre los tres grupos no fueron estadísticamente significativos.
ConclusiónLa dilatación de la pupila con una única gota de ciclopentolato al 1%, fenilefrina al 2,5% o tropicamida al 1% no está asociada a una disminución significativa de la profundidad de la cámara anterior o al estrechamiento de la ACA en los pacientes con SXF que presentan ángulos abiertos.
Exfoliation syndrome (XFS) is the most common identifiable cause of secondary open-angle glaucoma with a prevalence of more than 50% of cases of open-angle glaucoma in certain geographic locations.1,2 Patients with XFS need to be evaluated on a regular basis for the detection of glaucomatous optic neuropathy as elevated intraocular pressure (IOP) may result in a rapidly deteriorating visual function in these patients.1,3,4 Combined with an older age, the presence of XFS increases the probability of observing miotic pupils during clinical evaluation and making optic disc evaluations difficult to perform without pupillary dilatation.5 In clinical practice, pupillary dilatation is commonly performed using mydriatic or cycloplegic drops. Although one drop of short acting mydriatic agent such as 2.5% phenylephrine is sufficient for observing the posterior pole in eyes with light colored irides, darker brown-pigmented eyes may require a parasympatholytic agent such as 1% tropicamide or 1% cyclopentolate for adequate dilatation.6 Pupillary dilatation is not without its own risks in the elderly population; it may induce an episode of angle-closure glaucoma in susceptible individuals who have certain risk factors such as female gender, increased age, thickened lens, shallow anterior chamber and narrow angle configuration.7 The presence of XFS may compound these risk factors by inducing zonular laxity with resultant forward shift of the iris-lens diaphram and eventual increase in the degree of pupil block.1,8 In addition, narrow-angle configuration is more frequently observed in subjects with XFS as compared to age-matched control subjects.9,10
The aim of this study was to test the hypothesis that pupillary dilatation may induce narrowing of the anterior chamber depth (ACD) and anterior chamber angle (ACA) in subjects with XFS who present with clinically normal angle widths.
MethodsThe study was designed as a cross-sectional, observational study undertaken at a single university based hospital. The Tenets of the Declaration of Helsinki was followed throughout the study. Informed consent was obtained from all patients and the study was carried out with approval from the Institutional Review Board. Patients with clinical evidence of XFS were included this study. The diagnosis of XFS was made upon identification of fibrillogranular material at the pupillary ruff and/or the presence of typical anterior capsular appearance defined by the presence of central disc, clear intermediate zone and peripheral granular zone as observed with slit-lamp biomicroscopy. Clinical assessment of ACA width was made using the van Herick technique11 and gonioscopy. Only subjects with brown colored iridiae were included. Exclusion criteria consisted of intraocular pressure of >21mmHg on at least >2 office evaluations, ophthalmoscopic sign of glaucomatous optic nerve cupping, clinically evident narrow angles as defined by the presence of anterior chamber depth <1/2 of corneal thickness, iris bombe, visual field defects, pseudophakia, a history of intraocular surgery, glaucoma or posterior segment disease. Patients with mild to moderate grade of nuclear sclerosis were not excluded.
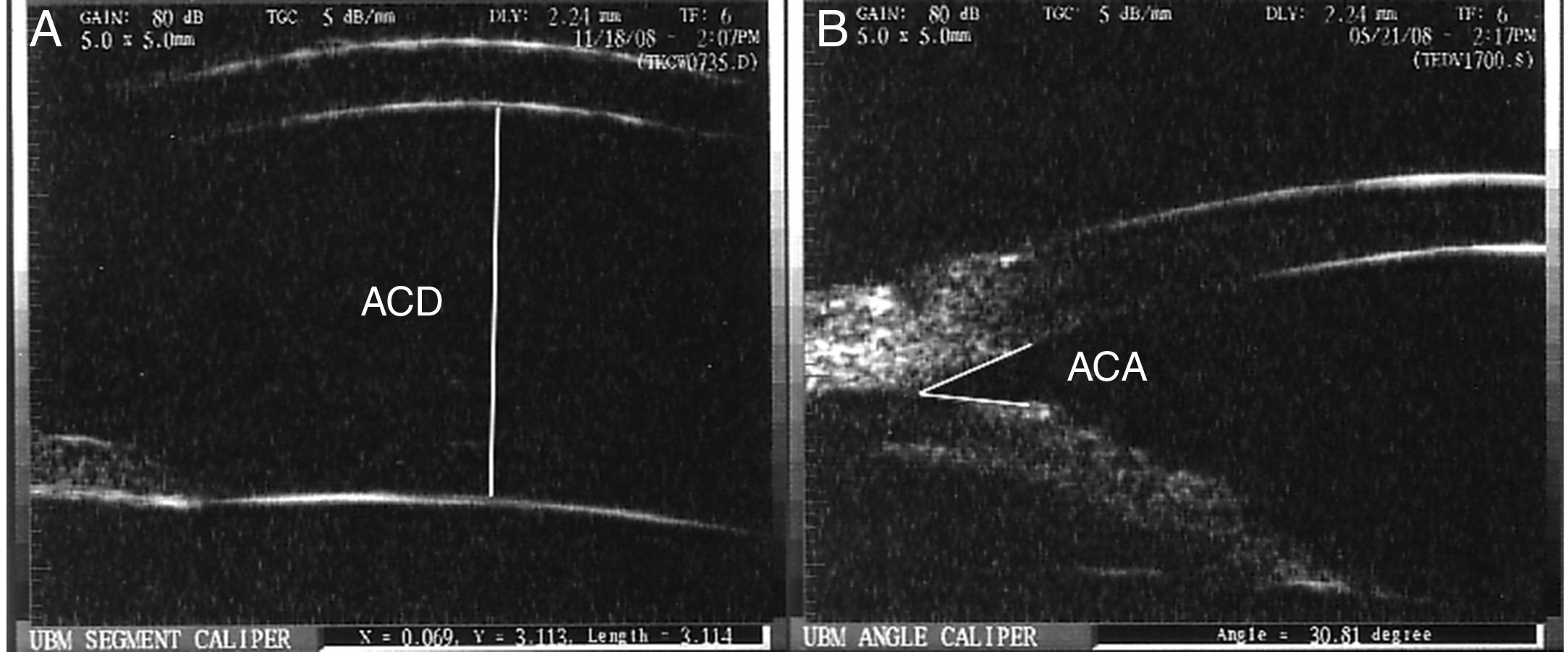
All patients underwent complete eye evaluation including slit-lamp biomicroscopy, gonioscopy and Goldmann applanation tonometry (with at least two measurements at different times of the day) and UBM. UBM was performed by a single observer (MCM) using Ultrasound Biomicroscopy Plus (Utah, USA) under the same light conditions. ACD was measured between the posterior boundary of the central cornea and the central portion of the anterior lens capsule (Fig. 1A). The average of two ACD measurements was calculated. The ACA width was defined as the angle formed by a line connecting the inner surface of the peripheral cornea and the iris root and a line joining the iris root and the peripheral anterior iris plane (Fig. 1B). The average of nasal and temporal ACA measurements was calculated. Measurements were performed with the caliper included in the UBM software. Patients were randomized to receive one drop of either 1% cyclopentolate (Group I; n=12), 2.5% phenylephrine (Group II; n=12) or 1% tropicamide (Group III; n=12) in the eye with XFS. Patients underwent repeat UBM evaluations and IOP determinations 40min following the instillation of topical drops. This time period was chosen to represent the instant at which maximum pharmacologic effect of the dilating agents would be observed.12 The maximum mydriatic effect of 1.0% cyclopentolate, 2.5% phenylephrine and 1% tropicamide are reported to range between 30–60min, 20–40min and 15–60min, respectively.12 The primary outcome measures were the changes in ACD and ACA following pupillary dilatation. The secondary outcome measure was the change in IOP between the same time points when anterior chamber parameters were evaluated. Patients were re-evaluated 24–48h following pharmacologic dilatation for signs of increased intraocular pressure.
(A) Anterior chamber depth (ACD) was determined by measuring the distance between the posterior surface of the central cornea and the most central portion of the anterior lens capsule. (B) Anterior chamber angle (ACA) width was measured as the angle formed between two lines coursing parallel to the anterior surface of the peripheral iris and peripheral portion of the posterior corneal surface.
The differences between preinstillation and postinstillation ACD [ACDΔ], ACA [ACAΔ] and IOP [IOPΔ] parameters were calculated for each subject. Statistical evaluations were performed using SPSS ver. 18.0 (Chicago, USA) software. Chi-square test was used to evaluate gender distribution within groups. The differences between predilatation and postdilatation values for ACD, ACA and IOP in all subjects were evaluated with the Wilcoxon signed rank test. The Kruskal–Wallis one-way analysis of variance test was employed to compare [ACDΔ], [ACAΔ] AND [IOPΔ] between Group I, Group II and Group III subjects. Mann Whitney U test was used to compare ACD, ACA and IOP values of female vs. male subjects. Spearman's correlation test was used to evaluate the strength of association between predilatation and postdilatation ACD, ACA and IOP parameters. A p value of less than 0.05 was considered significant.
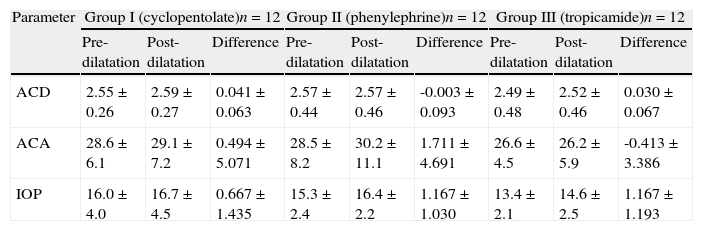
ResultsThirty-six eyes of 36 patients (20 females, 16 males) were included in the study. The mean age of the study subjects was 69.4±7.4 years (range=47–80 years). The average predilatation ACD, ACA and IOP values of all study subjects were 2.54±0.40mm, 27.9±6.3° and 14.9±3.1mmHg, respectively. There were no significant differences in either the age parameter (p=0.461), the female/male ratio (p=0.232), baseline ACD (p=0.841), ACA (p=0.761) or IOP (p=0.070) between the three groups. The pupils of all subjects were observed to be dilated more than 5mm and were nonreactive to light following application of mydriatic/cycloplegic drops. For all (n=36) subjects, the mean changes in ACD (0.03±0.08mm; p=0.109) and ACA (0.60±4.40°; p=0.520) following dilatation was not found to be statistically significant. The mean values related to ACA, ACD and IOP parameters before and after pupillary dilatation for the three groups are shown in Table 1. Comparing the three groups, dilatation induced changes in ACD [ACDΔ] (p=0.108), ACA [ACAΔ] (p=0.636) and IOP [IOPΔ] (p=0.160) were not significantly different as evaluated with the Kruskal–Wallis one-way analysis of variance test. In addition, [ACDΔ], [ACAΔ] and [IOPΔ] of subjects who received cycloplegic drops (Groups I and III) were not significantly different (p=0.052 for [ACDΔ], p=0.481 for [ACAΔ] and p=0.283 for [IOPΔ]) than subjects who received mydriatic drops (Group II).
The anterior chamber depth (ACD), anterior chamber angle (ACA) and intraocular pressure (IOP) measurements before and after pupillary dilatation.
| Parameter | Group I (cyclopentolate)n=12 | Group II (phenylephrine)n=12 | Group III (tropicamide)n=12 | ||||||
| Pre-dilatation | Post-dilatation | Difference | Pre-dilatation | Post-dilatation | Difference | Pre-dilatation | Post-dilatation | Difference | |
| ACD | 2.55±0.26 | 2.59±0.27 | 0.041±0.063 | 2.57±0.44 | 2.57±0.46 | -0.003±0.093 | 2.49±0.48 | 2.52±0.46 | 0.030±0.067 |
| ACA | 28.6±6.1 | 29.1±7.2 | 0.494±5.071 | 28.5±8.2 | 30.2±11.1 | 1.711±4.691 | 26.6±4.5 | 26.2±5.9 | -0.413±3.386 |
| IOP | 16.0±4.0 | 16.7±4.5 | 0.667±1.435 | 15.3±2.4 | 16.4±2.2 | 1.167±1.030 | 13.4±2.1 | 14.6±2.5 | 1.167±1.193 |
ACD: anterior chamber depth (mm); ACA: anterior chamber angle (°); IOP: intraocular pressure (mmHg).
None of the eyes in any group developed >10° of angle narrowing following pupillary dilatation and only three eyes in group I, and one eye in groups II&III had between 5° and 10° of angle narrowing following dilatation. In addition, only one eye in group II developed >5% (5.85%) of anterior chamber shallowing. Although there appeared to be a statistically significant mean IOP change of 1.0±1.2mmHg (p<0.001) for all subjects following dilatation, no significant intergroup IOP differences were detected (p=0.160).
The mean ACD and ACA measurements of male subjects (2.63±0.44mm, 28.4±7.2°) were not significantly different when compared to those of female (2.47±0.35mm, 27.5±5.7°) subjects (p=0.233, p=0.726).
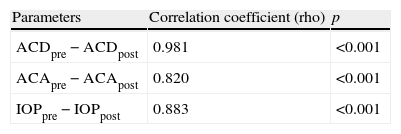
Spearman's correlation analysis did not reveal a significant association of subject age with predilatation ACD (r=−0.150; p=0.382), ACA (r=−0.06; p=0.743) or IOP (r=−0.071; p=0.683). However, predilatation and postdilatation values for ACD, ACA and IOP were found to be strongly correlated (Table 2).
Correlation analysis of anterior chamber depth, anterior chamber angle and intraocular pressure before and after pupillary dilatation as evaluated with Spearman's correlation analysis.
| Parameters | Correlation coefficient (rho) | p |
| ACDpre−ACDpost | 0.981 | <0.001 |
| ACApre−ACApost | 0.820 | <0.001 |
| IOPpre−IOPpost | 0.883 | <0.001 |
ACDpre: predilatation anterior chamber depth; ACDpost: postdilatation anterior chamber depth; ACApre: predilatation anterior chamber angle; ACApost: postdilatation anterior chamber angle; IOPpre: predilatation intraocular pressure; IOPpost: postdilatation intraocular pressure.
The reliability and repeatability of the ACA and ACD parameters as obtained with UBM were found to be high; the intraclass correlation coefficient and 95% confidence intervals (CI) of the ACD and ACA parameters were 0.999 (95% CI: 0.998–1.000) and 0.988 (95% CI: 0.967 and 0.996), respectively.
DiscussionPharmacologic pupillary dilatation has the potential to precipitate an acute attack of angle-closure glaucoma by creating a mid-dilated pupil configuration and pupillary block.13 This phenomenon is more likely in the setting of narrow angles or in eyes in which forward lens displacement occurs. The latter group includes eyes with ectopia lentis secondary to zonular dehiscence. Angle closure in eyes with XFS may develop due to the combination of factors including forward displacement of the lens-iris diaphram related to zonular weakness, lens thickening, formation of posterior synechiae and a rigid-thickened iris.8 In the current study, none of the eyes in any group developed >10° of angle narrowing following pupillary dilatation, suggesting that a single drop of commercially available dilating agents is safe for diagnostic pupillary dilatation in patients with XFS. This finding may be explained on the basis that one drop of topical dilating medications used in the study is not sufficient to induce alterations of the anterior chamber depth in XFS. Our results are representative of a subset of XFS patients with clinically open angles as evaluated with the van Herick method.
There exists a subset of patients with exfoliation syndrome who present with clinically overt narrow angles with or without iris bombe.8 In a recent study by Damji et al.,8; the ACD of XFS subjects with occludable angles (1.88±0.07mm) was lower compared to those with open-angle configuration (2.37±0.05mm). The average predilatation ACD and ACA values in the study subjects of our study were 2.54±0.40mm and 27.9±6.3°, respectively, more closely resembling the open-angle XFS group described in the study by Damji et al.8 In eyes with XFS, ACD may be an important parameter to evaluate the risk of angle closure and the presence of zonular weakness.8 We did not include subjects with clinically overt narrow angles in the current study in whom angle closure could have been precipitated with pupillary dilatation.
IOP elevations are reported in patients with glaucoma following pupillary dilatation.14 The suggested mechanisms that induce IOP elevations in glaucomatous eyes are pigment liberation into the anterior chamber with subsequent blockage of trabecular meshwork and decreased contractility of ciliary muscle on the trabecular meshwork leading to decreased aqueous outflow.14 The rise in IOP is observed within 45–120min following instillation of dilating drops and may remain elevated for 4–6h.14 Accordingly, pigment liberation into the anterior chamber is observed in XFS and may be accentuated with pupillary dilatation.1
In the current study, postdilatation ACD, ACA and IOP measurements were strongly correlated with their respective predilatation values (Table 2). This observation may have a potential value in making an estimate regarding anterior chamber parameters following pupillary dilatation. Although increasing age is associated with angle-closure glaucoma,15 it does not appear to be associated with angle width and anterior chamber depth in eyes with XFS.
A drawback of this study is that the anterior segment parameters of all subjects were evaluated only in the supine position due to the setup of the immersion lens of the UBM. Evaluation of ACD and ACA in the supine vs. prone position may be especially relevant in the presence of zonular weakness associated with XFS as measurements obtained in the supine position may not reflect the physiological state of eyes with XFS. ACD, ACA and IOP measurements were recorded only at the end of a 40-min interval; late measurements were not made. Therefore the protracted course of anterior chamber parameters cannot be inferred from the results of this study. The pigment liberation and IOP rise is reported to reach its maximum values at 1–2h following pupillary dilatation.16,17 IOP rise of 1.0mmHg was observed in patients with XFS as early as 40min post-dilatation; this may represent the beginning of the IOP rise associated with pigment liberation. However, patients were re-evaluated 24–48h following pharmacologic dilatation and no reports of ocular pain or blurring of vision was obtained from the subjects. Even though this study did not include a sample control group, the results of a recent study by Doganay et al. demonstrated that ACD and ACA are similar between subjects with XFS and healthy individuals.18 One final drawback of the study was that the three dilating drops could not be administered sequentially to all participants in different days due to the fact that several patients enrolled in the study lived in remote locations and could not return for repeat evaluations.
Narrow angle configuration in the setting of XFS is not uncommon; according to a previous report nearly one in three patients with XFS have narrow or occludable angles as verified with gonioscopic evaluation.9 As suggested by the findings of Damji et al.,8 eyes with XFS should be vigilantly evaluated for the presence of occludable angles using the van Herick technique followed by confirmatory compression gonioscopy if the corneal thickness/anterior chamber depth ratio is <0.25. In these cases, caution is always advised in performing pupillary dilatations in eyes with or without XFS. Nonetheless, pharmacologic pupillary dilatation for diagnostic purposes far outweigh the risk of inducing angle closure glaucoma.19 The results of our study suggest that pupillary dilatation with a single drop of 1.0% cyclopentolate, 2.5% phenylephrine or 1% tropicamide is not associated with narrowing of the anterior chamber angle in patients with XFS who have clinically open normal angle at presentation.
Conflicts of interestThe authors have no conflicts of interest to declare.