To compare the biocompatibility and subjective symptoms of four multipurpose solutions marketed in Palestine with hydrogel contact lenses.
Methods50 habitual soft contact lens wearers were recruited in this interventional crossover study. Subjects were asked to attend the optometry clinic five times. A new pair of hydrogel lenses (Bioxifilcon-B) were fitted each time. This pair was soaked randomly overnight in one of the following four-multipurpose solutions (NEOPLUS®, AvizorUnicaSensitive®, ReNuMultiPlus® and COMPLETERevitaLens®) which contain different disinfecting agents (PHMB, Phx, PAPB, and PQ-1+Alexidine, respectively), or non-preserved saline. At each visit, corneal staining, ocular redness and subjective symptoms were assessed.
ResultsThe percentage of corneal staining increased significantly (P≤0.050) after soaking the lenses with PHMB (86%), PAPB (64%) and Phx (32%) based-solutions. However, a non-significant increase was noticed after the use of PQ-1+Alexidine based solution (30%, P=0.083). Ocular redness evaluation showed a significant increase (P≤0.050) in limbal hyperemia after the use of all solutions, while bulbar redness was significantly increased after the use of biguanide-based solutions (P≤0.050). The subjective assessment analysis showed a non-significant change in comfort, dryness, photophobia and scratchiness (P≥0.050) at 2-h intervention using all solutions, except for the PHMB based solution which showed a significant change in subjective symptoms (P≤0.050).
ConclusionThe combination of Bioxifilcon-B hydrogel contact lenses and solution containing PHMB, PAPB and Phx-disinfectants induced a significant increase in corneal staining after 2h of CL-wear with a higher severity when the PHMB-based solution was used, while the PQ-1+Alexidine-based solution did not. Only the PHMB-based solution triggered a significant change in subjective symptoms which might which suggests that it might be related to the severity of staining rather than the induction of staining.
Comparar la biocompatibilidad y los síntomas subjetivos de cuatro soluciones multiusos comercializadas en Palestina con lentillas de hidrogel.
MétodosEn este estudio intervencionista cruzado, reunimos a 50 usuarios habituales de lentillas blandas. Solicitamos a los sujetos que acudieran cinco veces a la clínica optométrica. Cada vez ajustamos un nuevo par de lentillas de hidrogel (Bioxifilcon-B). Dicho par se sumergió aleatoriamente por la noche en una de las siguientes soluciones multiuso (NEOPLUS®, AvizorUnicaSensitive®, ReNuMultiPlus® y COMPLETERevitaLens®), que contienen diferentes agentes desinfectantes (PHMB, Phx, PAPB, y PQ-1+Alexidina, respectivamente), o solución salina sin conservantes. Durante cada visita, valoramos la coloración de la córnea, el enrojecimiento ocular y los síntomas subjetivos.
ResultadosEl porcentaje de coloración de la córnea se incrementó significativamente (P≤0,05) tras sumergir las lentillas en soluciones basadas en PHMB (86%), PAPB (64%) y Phx (32%). Sin embargo, se observó un incremento no significativo tras utilizar la solución basada en PQ-1+Alexidina (30%, P=0,083). La evaluación del enrojecimiento ocular reflejó un incremento significativo (P≤0,05) de la hiperemia limbal tras el uso de todas las soluciones, mientras el enrojecimiento bulbar se incrementó significativamente tras utilizar soluciones basadas en biguanida (P≤0,05). El análisis de valoración subjetiva reflejó un cambio no significativo en cuanto a comodidad, sequedad, fotofobia y picazón (P≥0,05) durante la intervención de dos horas utilizando todas las soluciones, exceptuando la solución basada en PHMB, que reflejó un cambio significativo en cuanto a síntomas subjetivos (P≤0,05).
ConclusiónLa combinación de las lentillas de hidrogel Bioxifilcon-B y la solución con contenido de desinfectantes PHMB, PAPB y Phx indujo un incremento significativo de la coloración de la córnea tras 2h de uso de lentillas, con una severidad superior al utilizarse la solución basada en PHMB, hecho que no se produjo con la solución basada en PQ-1+Alexidina. Únicamente la solución basada en PHMB desencadenó un cambio significativo en cuanto a síntomas subjetivos, lo cual podría sugerir que podría guardar relación con la severidad de la coloración, en lugar de la inducción de la misma.
A large tendency towards using planned replacement soft contact lenses (CL) is found in the Middle East1,2 and all over the world.3–5 In consequence, disinfecting care system must be used to keep the ocular surface and CL healthy and free of infections. Currently, the lens care market is dominated by the use of multi-purpose solutions (MPS)6 which are used to clean, disinfect, rinse and store CL.6 MPS are composed of many ingredients such as water, surfactants, wetting agents, buffers, chelating agents, and disinfectants.6
In spite of the positive effect of the contact lens care products, they could produce corneal epithelium punctuate staining when combined with soft lenses. This phenomenon is known as solution induced corneal staining (SICS). It is attributed to the epithelial cell damage caused by the contact between MPS and the ocular surface.7,8 The SICS reaction is transient and it is mostly evident after 2–4h of CL wear.7 Further, it has been suggested that SICS is dependent on the interaction between the CL material and the solution components,9–11 mainly the preservatives.9 The greatest extent of SICS was found when biguanide-based solutions are used. Examples of biguanide derivatives are polyhexamethylene biguanide hydrochloride (PHMB) and polyaminopropyl biguanide (PAPB).7,10,11 Additionally, a recent study suggested that other solution components such as the surfactants play an important role in triggering corneal staining.12
SICS may have a negative impact on the ocular surface, increasing the risk of corneal inflammation by three times,13 increasing the risk of corneal erosion due to a poor surface wettability,14 and decreasing the CL wear comfort15 which is the primary reason for CL discontinuation.16 However, other research studies have found no relation between SICS and discomfort.10,17
It is common practice to advice the patient formerly of the best combination. However, no such information is available in Palestine. Therefore, this interventional study was conducted to evaluate the biocompatibility and subjective assessment of four marketed MPS's in Palestine, using one hydrogel CL (45% Bioxifilcon B and 55% H2O). The contact lens and MPS used in this study were chosen as they are the most commonly used within the Palestinian community. Additionally, hydrogel CL's were found to be more prescribed (60.3%) than silicon hydrogel lenses (31.3%) and RGP lenses (8.4%) in a nearby country1 which might be due to its lower cost. The corneal staining, ocular redness and subjective symptoms were assessed prior CL wear and after 2h of CL wear, knowing that SICS is more evident after 2–4h of CL wear.7
MethodsStudy designFifty habitual soft contact lens wearers from both genders were recruited in this interventional, double-masked crossover study. The biocompatibility of four MPS in conjugation with a hydrogel soft CL (45% Bioxifilcon B and 55% H2O, Morning-Q Ultra Soo 55, INTEROJO, Gyeonggi-do, Korea) was assessed and compared to a control preservative-free Saline solution (CooperVision, New York, United Sates). The tested MPS are NEO PLUS® (Neo Vision, Gangwon-do, Korea), AvizorUnicaSensitive® (Avizor, Torrejon De Ardoz, Spain), ReNuMultiPlus® (Bausch & Lomb, Kingeston-upon-Thames, UK) and COMPLETE Revita Lens® (Abbott Medical Optics, Dublin, Ireland), which contain different disinfecting agents (polyhexamethylene biguanide hydrochloride (PHMB), polyhexanide (Phx), polyaminopropyl biguanide (PAPB) and polyquaternium-1 (PQ-1)+Alexidine, respectively.
This study was conducted in compliance with the tenets of the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board (IRB) committee at An-Najah National University. All subjects received a written participant information sheet in Arabic language to ensure they fully understand the objectives, procedure and the significance of the research. In addition, an informed consent was obtained from all participants prior the start of the study.
Eligible participants were asked to visit the optometry clinic at An-Najah National University six times (one initial visit and five assessment visits). Each visit lasted for 15±5min. The initial visit was conducted to illustrate the nature of the study and to check the eligibility of the participants based on the inclusion and exclusion criteria.
Successful soft CL wearers who were at least 18 years old, with best-corrected visual acuity equal or better than 0.2logMAR and with healthy eyes and clear corneas were recruited in this study. Participants who were pregnant, lactating, affected by an active ocular surface disease or abnormalities, or have known hypersensitivity to any of the CL solutions used in this study were excluded. Additionally, the ocular health of the participants was checked at the initial visit. Participants with corneal staining ≥type 2 staining in any of the corneal regions or ≥20% corneal staining in one or more corneal region or conjunctival injection >grade 1 in either eye were also excluded.
Assessment visitSubjects were asked to avoid wearing contact lenses for at least 12h prior all five assessment visits. At the beginning of each assessment visit an ocular examination to check their visual acuity, anterior eye segment health and corneal staining was performed. Additionally, participants were asked to subjectively assess a number of symptoms such as discomfort, photophobia, dryness, scratchiness, burning, and stinging.
A new pair of contact lenses was dispensed at each visit which was pre-soaked overnight (10–12h) in one of the trial solutions in a randomised order. The subjects were asked to wear the CL and return to the clinic after 2h to repeat the ocular evaluation and the subjective assessment prior the CL removal.
Participants were asked to return to their original contact lenses and lens care regimen or spectacles afterwards. The second visit was then scheduled, after 1–2 days, in according to the participant's availability.
Ocular examination proceduresSlit lamp biomicroscope (TOPCON, Tokyo, Japan) was used to assess the ocular redness and corneal staining prior wearing contact lenses and after 2h of CL wear. The grade of ocular redness (limbal and bulbar) was graded from 0 to 4 in 0.5 steps according to EFRON Grading scale (Butterworth-Heinemann, Oxford, UK) where “0” corresponds to no redness and “4” corresponds to severe redness.
The cornea was then stained by a Ful-Glo® (fluorescein sodium ophthalmic strips, USP) (Akorn, Inc., Lake Forest, Illinois, United States) moistened with one drop of preservative-free saline. The staining in each of the five corneal regions (central, superior, inferior, nasal and temporal) was assessed in terms of area size (extent) and type (severity). The staining extent was graded in a 0–100 percentage scale where 0 corresponds to no area stained and “100” corresponds to fully distributed staining. The staining severity was graded from 0 to 4 in 0.5 steps according to EFRON grading scale where “0”corresponds to no staining and “4” corresponds to patch staining.
Subjective assessmentSubjective symptoms including discomfort, photophobia, dryness, scratchiness, burning and stinging were assessed using a 5-points Likert-style scale.18 Participants were asked to respond to the following statements: my eyes are un-comfortable, my eyes are sensitive to light, my eyes are dry, my eyes scratch and my eyes burn and sting, where one of the following answers can be chosen; strongly agree, agree, undecided, disagree or strongly disagree. These responses were then converted to a numerical scale (0–4, respectively) for analysis purposes.
Statistical analysisThe Statistical Package of Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA) was utilised for data entry and statistical analysis. Based on medium size effect and alpha level of 0.050, this study provided an 80% or greater power to detect changes in corneal staining, redness and subjective symptoms, assuming data from 50 participants were analysed. The worse eye with highest normalised staining and redness (baseline data was subtracted), or the right eye if staining is equal in both eyes was included in data analysis. The proportion of participants presented with solution-induced corneal staining (staining of ≥grade 1 in 4 or 5 corneal regions)19 after 2h of CL wear was evaluated. Additionally, the mean of corneal staining (area size and severity), limbal redness and bulbar redness were assessed. Subjective symptoms (baseline date was subtracted) of discomfort, photophobia, dryness, scratchiness, burning, and stinging were also evaluated. Paired analysis of SICS, staining extent and grade, bulbar and limbal redness, in addition to subjective symptoms at baseline vs. 2h of CL wear were conducted using Wilcoxon's signed rank test. It was also used to compare the results of the test MPS and the saline control solution. Additionally, analysis of repeated measures using Friedman's test and Post hoc analysis with Wilcoxon signed-rank test with a Bonferroni correction were utilised to assess differences between all solutions. Correlations between corneal staining, ocular redness and subjective symptoms were assessed with the Pearson r coefficient of correlation. A P-value ≤0.050 were considered statistically significant.
ResultsA total of 50 participants were enrolled in this study, where 30 subjects (60%) were females and 20 subjects (40%) were males. The average age was 21.2±1.5 years (ranging from18 to 30 years), and the average spherical equivalent was −3.07±1.85D (ranging between −8.00D and +1.00D), see Table 1.
Demographics and baseline characteristics of the participants.
| Demographics and baseline characteristics (n=50) | |
|---|---|
| Age | Years |
| Mean±SD | 21.2±1.5 |
| Gender | No. (%) |
| Female | 30 (60%) |
| Male | 20 (40%) |
| Habitual lenses | No. (%) |
| Silicone hydrogel lenses | 6 (12%) |
| Conventional hydrogel lenses (Monthly replacement) | 19 (38%) |
| Unclear | 25(50%) |
| Spherical equivalent | Diopter |
| Mean±SD | −3.07±1.85 |
| Habitual solution | No. (%) |
| NEO PLUS® | 23 (46%) |
| AvizorUnicaSensitive® | 10 (20%) |
| COMPLETE® | 8 (16%) |
| ReNuMultiPlus® | 3 (6%) |
| DISPO® | 2 (4%) |
| Saulfon (all in one light) | 1 (2%) |
| Others | 1 (2%) |
| None | 2 (4%) |
| Cleaning agent | No. (%) |
| PHMB | 23 (26%) |
| PAPB | 3 (6%) |
| PQ-1+Alexidine | 8 (16%) |
| Phx | 13 (26%) |
| Others | 1 (2%) |
| None | 2 (4%) |
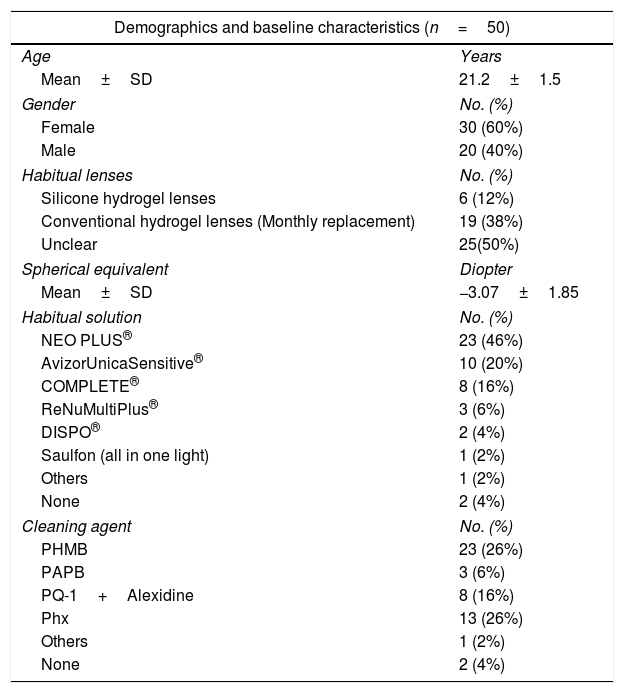
The percentage of eyes with SICS (corneal staining ≥grade I in 4 or 5 regions of the cornea)19 increased significantly after 2h of CL wear soaked in Avizor UnicaSensitive® (Phx) (32.0%, P=0.020), ReNuMultiPlus® (PAPB) (64.0%, P<0.001) and NEO PLUS® (PHMB) (86.0%, P<0.001) when compared to SICS percentage at baseline (00.0%). On the other hand, this change was not significant when COMPLETE® (PQ-1+Alexidine) (30.0%) or the control solution, saline (00.0%) were used (Fig. 1).
Percentage of eyes with SICS after 2h of contact lens wear with tested solutions (n=50). No eyes had an indication of SICS (corneal staining ≥grade I in 4 or 5 regions of the cornea) at baseline. P-values compared percent of eyes with SICS at 2h intervention and at baseline (00.0%). PAPB, polyaminoprpyl biguanide; PHMB, polyheamethylene biguanide hydrochloride; Phx, polyhexanide; PQ-1, polyquaternium-1.
Additionally, in comparison with the saline control solution, a statistical significant difference (P≤0.050) in SICS was found using all test solutions after 2h of CL wear.
Comparison of the repeated measures showed a statistically significant difference in SICS among the test solutions after 2h of CL wear. Differences between solutions showed a non-significance difference between NEO PLUS® & ReNuMultiPlus®, and between COMPLETE® & AvizorUnicaSensitive®. However, significant differences (P≤0.050) between NEO PLUS® solution and COMPLETE® & AvizorUnicaSensitive® solutions were found with a higher mean rank for the NEO PLUS®. Additionally, significant differences (P≤0.050) between ReNuMultiPlus® and COMPLETE® & AvizorUnicaSensitive® were found with a higher mean rank for ReNuMultiPlus®.
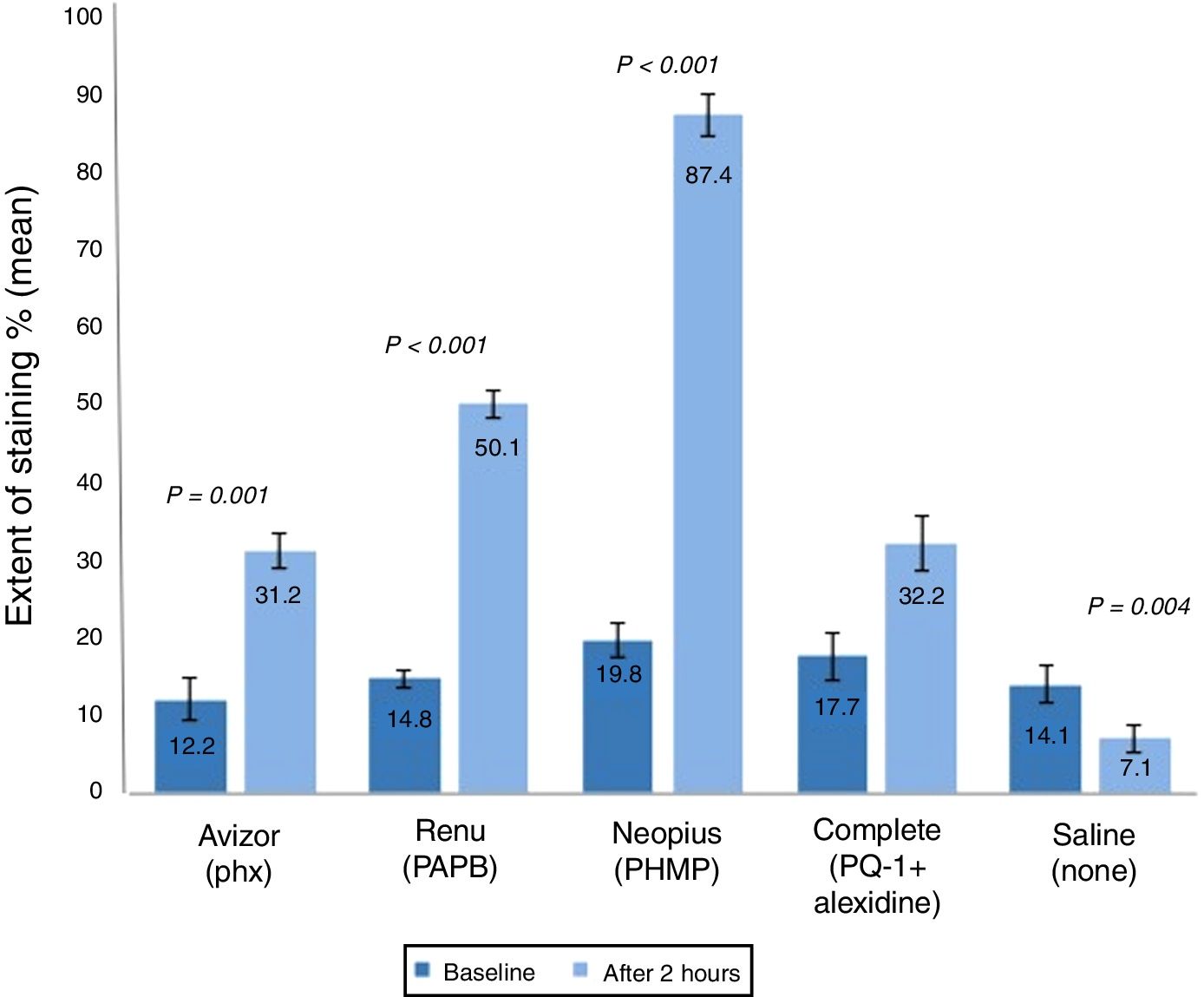
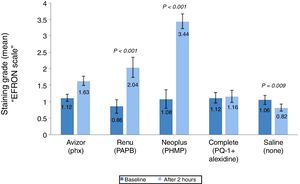
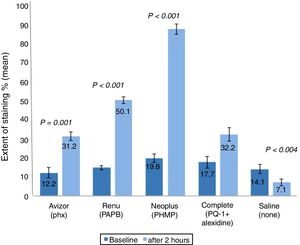
The corneal staining severity was also analysed in terms of area and grade (Figs. 2 and 3). The stained area increased significantly after 2h of CL wear soaked in AvizorUnica Sensitive® (Phx) (by 19.0%, P=0.001), ReNuMultiPlus® (PAPB) (35.3%, P<0.001), and NEO PLUS® (PHMB) (by 67.6%, P<0.001), when compared to baseline. While this increase was not significant when COMPLETE® (PQ-1+Alexidine) was used (by 14.5%). The use of saline solution showed a significant decrease in staining area (by 7.0% P=0.004) (Fig. 2).
Extent of corneal staining (mean±SD) after 2h of contact lens wear with tested solutions (n=50). P-values compared the extent of corneal staining after 2h of CL wear and at baseline. PAPB, polyaminoprpyl biguanide; PHMB, polyheamethylene biguanide hydrochloride; Phx, polyhexanide; PQ-1, polyquaternium-1.
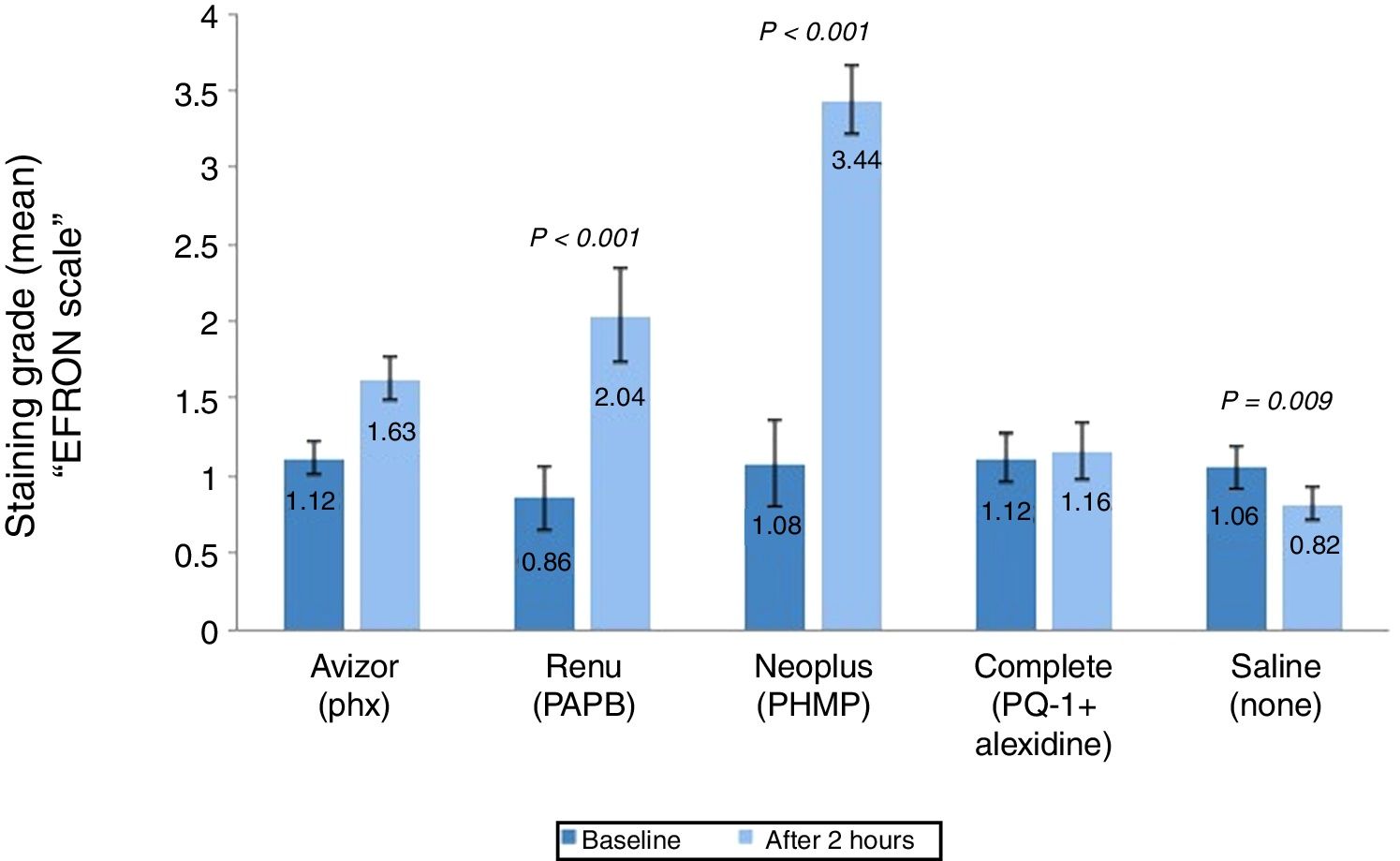
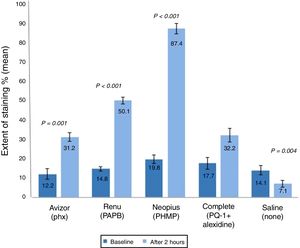
Grade of corneal staining (mean±SD) after 2h of CL wear with tested solutions (n=50). P-values compared the grade of corneal staining severity after 2h of CL wear and at baseline. PAPB, polyaminoprpyl biguanide; PHMB, polyheamethylene biguanide hydrochloride; Phx, polyhexanide; PQ-1, polyquaternium-1.
Additionally, in comparison with the saline control solution, a statistical significant difference (P<0.001) in corneal staining extent after 2h of CL wear was found using all test solutions.
Corneal staining grade was also analysed (Fig. 3) and found to be increased significantly after 2h of CL wear by (1.18, P<0.001) and (2.36, P<0.001) soaked in ReNuMultiPlus® (PAPB) and NEO PLUS® (PHMB), respectively, when compared to baseline. While this increase was not significant by 0.51 and 0.04 when AvizorUnica Sensitive® (Phx) and COMPLETE® (PQ-1+Alexidine) were used, respectively. The use of the saline control solution showed a significant decrease in staining grade (by 0.24 P=0.009) (Fig. 3).
Additionally, in comparison with the saline control solution, a statistical significant difference in corneal staining grade after 2h of CL wear was found when ReNuMultiPlus® (PAPB) (P<0.001) and NEO PLUS® (PHMB) (P<0.001). No significant difference was found when AvizorUnica Sensitive® (Phx) and COMPLETE® (PQ-1+Alexidine) were used.
Comparison of the repeated measures showed statistically significant differences in corneal staining extent and severity among the test solutions after 2h of CL wear. Differences between solutions showed a significance difference in corneal staining (extent & severity) between NEO PLUS® solution and the other test solutions (all P≤0.050).
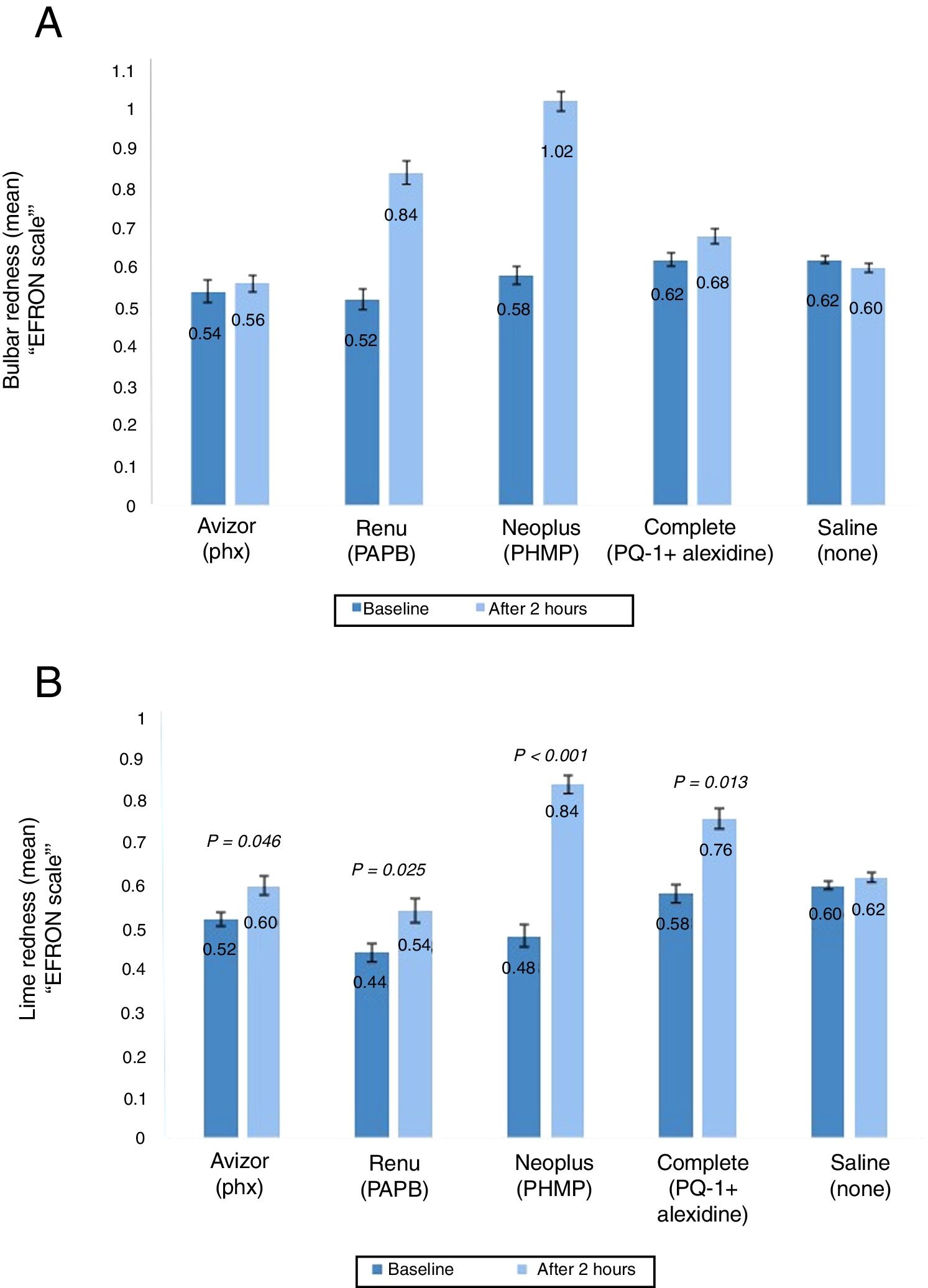
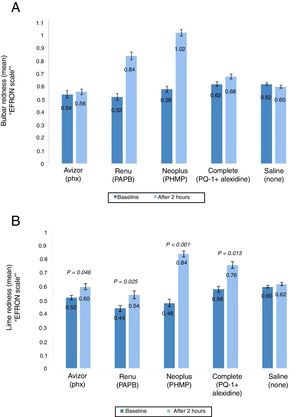
Ocular rednessThe ocular redness (bulbar and limbal redness) was assessed prior CL wear and after 2h (Fig. 4A and B). The change in mean bulbar redness score was increased significantly after 2h of CL wear when ReNuMultiPlus® (PAPB) (by 0.32, P<0.001), and NEO PLUS® (PHMB) (by 0.44, P<0.001) were used, when compared to baseline. While this increase was not significant when AvizorUnica Sensitive® (Phx) (by 0.04) and COMPLETE® (PQ-1+Alexidine) (by 0.06) were used. The use of the saline control solution showed a non-significant decrease in bulbar redness by 0.02 (Fig. 4A). Additionally, in comparison with the saline control solution, a statistical significant difference in bulbar redness after 2h of CL wear was found when ReNuMultiPlus® (PAPB) (P=0.050) and NEO PLUS® (PHMB) (P=0.001) were used. No statistical significant difference was found when AvizorUnica Sensitive® (Phx) and COMPLETE® (PQ-1+Alexidine) were used.
The change in bulbar redness (A) and limbal redness (B) score (mean±SD) between baseline and after 2h of CL wear with tested solutions (n=50). P-values compared the extent of bulbar and limbal redness after 2h of CL wear and at baseline. PAPB, polyaminoprpyl biguanide; PHMB, polyheamethylene biguanide hydrochloride; Phx, polyhexanide; PQ-1, polyquaternium-1.
The change in limbal redness score after 2h of CL wear was increased significantly (P≤0.050) by 0.08, 0.10 and 0.18 when AvizorUnica Sensitive®, ReNuMultiPlus®, NEO PLUS® and COMPLETE® were used, respectively, when compared to baseline data. The use of the saline control solution showed a non-significant increase in limbal redness (by 0.02) (Fig. 4B).
Additionally, in comparison with the saline control solution, a statistical significant difference in limbal redness after 2h of CL wear was found when NEO PLUS® (PHMB) (P=0.041) was used. No statistical significant difference was found when ReNuMultiPlus® (PAPB), AvizorUnica Sensitive® (Phx) and COMPLETE® (PQ-1+Alexidine) were used.
Comparison of the repeated measures was performed. Although there was an overall significant difference in bulbar and limbal redness among the MPS used, no two test solutions were significantly different from one another in pairwise comparison after 2h of CL wear (all P≥0.050).
Subjective assessmentThe participants’ assessment of discomfort, photophobia, dryness, scratchiness, burning and stinging prior wearing the lenses and after 2h of CL wear were analysed before CL wear and after at 2h of CL wear, Table 2.
The mean±SD of subjective assessment of discomfort, photophobia, dryness, scratchiness, burning and stinging at baseline and after 2h of CL wear. Score is from 0 to 4; 0: lowest, 4: highest.
| MPS | Discomfort | Photophobia | Dryness | Scratchiness | Burning and stinging | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 2h | P-value | Baseline | After 2h | P-value | Baseline | After 2h | P-value | Baseline | After 2h | P-value | Baseline | After 2h | P-value | |
| AvizorUnica Sensitive® | 3.560±0.787 | 3.580±0.810 | 0.877 | 3.340±0.842 | 3.460±0.730 | 0.063 | 3.320±0.935 | 3.440±0.861 | 0.290 | 3.580±0.731 | 3.680±0.587 | 0.288 | 3.540±0.831 | 3.740±0.643 | 0.041 |
| ReNuMultiPlus® | 3.600±0.463 | 3.700±0.756 | 0.259 | 3.540±0.758 | 3.580±0.838 | 0.414 | 3.160±0.131 | 3.360±0.177 | 0.385 | 3.800±0.370 | 3.840±0.452 | 0.480 | 3.120±0.551 | 3.640±0.239 | 0.026 |
| NEO PLUS® | 2.660±0.642 | 3.580±0.507 | 0.001 | 3.140±0.931 | 3.520±0.903 | 0.017 | 2.940±0.107 | 3.280±0.268 | 0.050 | 3.240±0.658 | 3.660±0.980 | 0.026 | 2.300±0.722 | 3.640±0.607 | 0.001 |
| COMPLETE® | 3.560±0.578 | 3.620±0.824 | 0.627 | 3.560±0.481 | 3.580±0.622 | 0.799 | 3.200±0.705 | 3.340±0.519 | 0.322 | 3.640±0.219 | 3.700±0.374 | 0.261 | 3.600±0.603 | 3.640±0.485 | 0.100 |
| Saline® | 3.640±0.525 | 3.580±0.609 | 0.063 | 3.680±0.782 | 3.600±0.587 | 0.125 | 3.560±0.961 | 3.340±0.644 | 0.043 | 3.740±0.443 | 3.720±0.454 | 0.317 | 2.470±0.471 | 2.140±0.706 | 0.039 |
The numbers in bold indicates the change here is statistically significant.
All subjective symptoms were significantly worse (P≤0.050) after 2h of CL wear when NEO PLUS® (PHMB) was used, while this increase was non-significant (P≥0.050) when COMPLETE® (PQ-1+Alexidine) was used. The use of AvizorUnicaSensitive® (Phx) and ReNuMultiPlus® (PAPB) showed a non-significant increase (P≥0.050) in discomfort, photophobia, dryness and scratchiness and a significant increase (P≤0.050) in stinging and burning sensation after 2h of CL wear. The saline control solution showed no statistical change (P≥0.050) in discomfort, photophobia, scratchiness, dryness, but a significant improvement (P≤0.050) in dryness and burning and stinging symptoms after 2h of CL wear (Table 2).
Additionally, in comparison with the saline control solution, a statistical significant difference in subjective symptoms after 2h of CL wear was found only when NEO PLUS® (PHMB) (P=0.034) was used, while a non-statistical significant difference (P≥0.050) was found when the other test solutions were used.
Comparison of the repeated measures showed a statistically significant differences in subjective symptoms among the test solutions after 2h of CL wear. Differences between solutions showed a significance difference in subjective symptoms between NEO PLUS® solution and the other test solutions (all P≤0.050).
Overall, corneal staining and redness did not show a correlation with subjective symptoms (r=−0.11 to −0.20, P≥0.05).
DiscussionIn this study, a higher increase in staining was found when biguanide derivative solutions (NEO PLUS® and ReNuMultiPlus®) were used. This finding confirms previous studies7,10,11,17 which reported a significant increase in corneal staining when PHMB-preserved solution was used, but by different percentages. This could be explained by several factors. Firstly, the use of different concentration and different CL materials in these studies could affect the absorbance and release of preservatives quantity, and therefore induced different percentages of SICS.19 Secondly, it was proven that other MPS’ components might be involved in inducing SICS such as the surfactants.12 Lastly, the presence of different lubricating agents in each MPS could affect the development of SICS.10 On the other hand, another study reported a similar increase in staining between solutions containing PHMB and other solutions containing different antimicrobial agents when combined with silicon hydrogel CL.20 This might be due to the reduced ability of silicon hydrogel lenses to uptake and release PHMB when compared with traditional hydrogels. Furthermore, in this study, a lower percentage of SICS and no change in cornel staining grade was found when Phx-preserved solution (AvizorUnica Sensitive®) was used. This might be due to its effective lubricant agent (Sodium hyaluronate) which is known of providing hydration and comfort.10 The other test solutions in this study do not have lubrication agents.
Bulbar redness was found to be increased significantly, compared to baseline and control solution, only when biguanide derivative solutions (NEO PLUS® and ReNuMultiPlus®) were used. This finding aligns with what previous studies found.7,10 Furthermore, all four used solutions were found to induce limbal redness significantly, after 2h of CL wear, where PHMB-based solution (NEO PLUS®) showed the highest increase compared to baseline and control solution. None of the solutions showed an increase in mean rank in pairwise comparisons, and no change in limbal redness was also found when saline was used. This might suggest that limbal redness could be partially related to the presence of the CL on the eye.7,10
Subjective symptoms including discomfort, photophobia, dryness, scratchiness, burning and stinging were significantly worse when PHMB-based solution (NEO PLUS®) was used. NEO PLUS MPS was also found to induce a significant increase in corneal staining and ocular redness. However, AvizorUnica Sensitive® (Phx) and ReNuMultiPlus® (PAPB) induced a statistically significant increase in SICS but showed no change in subjective symptoms (discomfort, dryness, photophobia and scratchiness). On the other hand, no change in corneal staining and subjective symptoms was found when COMPLETE® (PQ-1+Alexidine) was used. In this study, no correlation was found between corneal staining and subjective symptoms. This finding supports previous research10 and contraindicate with others20 which found a positive correlation between SICS and discomfort after 2h of CL wear. This could suggest that SICS is not alone responsible for the increase in subjective symptoms, and other factors might have a role. Some of these factors include: the different contact lenses materials, different concentration of disinfectants, tear film healthiness and different lubrication agents used.10,19 Moreover, it is worth mentioning that a significant increase in discomfort was noticed when the severity of staining grade was higher after the use of NEO PLUS® MPS when compared to baseline. This may suggest that discomfort might be more related to the severity of corneal staining rather than the induction of it.
In this study, the control saline solution exhibited no change in SICS and bulbar redness, in addition to a significant decrease in corneal staining extent and grade. A significant decrease in the sensation of dryness, stinging and burning was also found after 2h of CL wear. This may be due to the non-preservative nature of the used saline and to the possible protection of the CL to the ocular surface from the environment. This finding may also suggest that rinsing the CL with saline prior CL insertion could minimise ocular staining and redness and improve subjective symptoms. However, a previous study11 found that rinsing the CL with non-preserved saline did not affect the level of SICS. The grade and extent of corneal staining in addition to ocular redness were not assessed in this study11 which might be minimised by rinsing the lens with non-preserved saline prior CL insertion.
Since SICS is influenced by the interaction between the CL material and solution components, this study is limited by the use of one type of contact lens material. However, Bioxifilcon B contact lenses are dominated by the Palestinian market and there was a necessary to study the biocompatibility of this material with commonly used MPS in this community. Finally, the ocular assessment and subjective symptoms were only evaluated after 2h of CL wear in this study and not providing data beyond this time frame. However, a previous study showed that SICS return to its baseline after 6h of wear.7 In future research, other CL material can be included with a longer follow up period to study the effect of different combination on corneal staining, eyelid redness and roughness as it can be affected by MPS21 in addition to subjective symptoms.
ConclusionIn conclusion, this study showed that the combination of Bioxifilcon B hydrogel CL with NEO PLUS® presents more subjective symptoms (discomfort, dryness, photophobia, scratchiness, stinging and burning) and ocular surface response (redness, staining, SICS) than with AvizorUnica Sensitive®, ReNuMultiPlus® and COMPLETE®. Choosing the appropriate CL material and solution (which has an effective lubricant agent) for CL patients formerly would minimise the overall risks of SICS and maintain healthy CL experience.
FundingThis work was funded by the Deanship of Scientific Research at An-Najah National University [grant number ANNU1718Sc002, 2017].
Conflicts of interestThe authors have no conflicts of interest to declare.
The financial support of An-Najah National University to undertake this work under grant number (ANNU1718Sc002,2017) is highly acknowledged.