To investigate immediate and short-term visual recovery in a large cohort of 2093 myopic eyes (with or without astigmatism) treated with SmartSurfACE procedure, a combination of Transepithelial Photo Refractive Keratectomy (PRK) and Smart Pulse Technology (SPT, SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany).
MethodsIn this retrospective case series, post-operative outcomes were evaluated immediately after the surgery (Day 0), at day 1 and 3 months postoperatively, after myopic SmartSurfACE treatment with mean pre-operative spherical equivalent -4.65±2.53D and range from −16.13D to −0.13D. In all cases, pre and postoperative standard examinations were performed. The analysis evaluated preoperative Corrected and Uncorrected Distance Visual Acuity (CDVA and UDVA, respectively), and postoperative UDVA, monocularly and binocularly, immediately after the surgery, at day 1 and 3 month follow up.
ResultsSixty-two percent eyes achieved monocular UDVA 20/40 or better immediately after the surgery, while 82% patients achieved binocular UDVA 20/32 or better immediately after the surgery. At 3-month postoperatively, monocular UDVA 20/25 or better was achieved in 94% eyes. Treated eyes achieved immediately after the surgery or by the next day mean UDVA 20/41±8. UDVA improved significantly from Day 1 to 3-months follow up (p<0.0001 for both OS and OD) to mean UDVA 20/21±5 (equal to preoperative CDVA 20/21±8).
ConclusionImmediate and short-term visual recovery after SmartSurfACE PRK in our large cohort was rapid, providing functional binocular UDVA immediately after the surgery.
Investigar la recuperación visual inmediata y a corto plazo en una amplia cohorte de 2.093 ojos miópicos (con o sin astigmatismo) tratados con el procedimiento SmartSurfACE, una combinación de Queratectomía Fotorrefractiva Transepitelial (PRK) y SPT (Smart Pulse Technology, SCHWIND eye-tech-solutions GmbH, Kleinostheim, Alemania).
MétodosEn esta serie de casos retrospectivos, se evaluaron los resultados post-operatorios inmediatamente tras la cirugía (Día 0), al día siguiente a la misma, y a los tres meses de la intervención, tras el tratamiento de la miopía con SmartSurfACE, con un equivalente esférico preoperatorio medio -4,65±2,53D y rango desde -16,13D a -0,13D. En todos los casos se realizaron exámenes estándar preoperatorios y postoperatorios. El análisis evaluó preoperatoriamente la agudeza visual de lejos corregida y no corregida (CDVA y UDVA), y postoperatoriamente UDVA, monocular y binocular, inmediatamente tras la cirugía, al día siguiente, y a los tres meses de seguimiento.
ResultadosEl 62% de los ojos logró UDVA monocular 20/40 o un valor mejor inmediatamente tras la cirugía, y el 82% de los pacientes logró UDVA binocular 20/32 o un mejor valor inmediatamente tras la cirugía. A los tres meses de la intervención, se logró UDVA monocular 20/25 o un valor mejor en el 94% de los ojos. Los ojos tratados lograron inmediatamente tras la cirugía o al día siguiente UDVA 20/41±8. UDVA mejoró significativamente entre el día siguiente y los tres meses de seguimiento (p<0,0001 para ambos ojos) a un valor medio de 20/21±5 (igual a CDVA preoperatoria de 20/21±8).
ConclusiónLa recuperación visual inmediata y a corto plazo tras PRK con SmartSurfACE en nuestra amplia cohorte fue rápida, logrando una UDVA binocular funcional inmediatamente tras la cirugía.
Several parameters characterizing the laser beam are critical for an accurate and safe laser refractive surgery.1–4 Challenges remain in achieving higher ablation smoothness, with the temporal and spatial distribution of the laser spots (scan sequence) showing an influence on the surface quality and maximum ablation depth of the ablation profile. Smoothness of ablation may also vary with different excimer lasers systems.5 Simulation models have shown that the beam characteristics used in a corneal laser procedure have a major impact on the postoperative surface quality; and optimum laser characteristics have been identified.6
Smoother corneal surfaces postoperatively have related advantages: (1) Short term outcomes may be better in the period where the epithelium remodelling/smoothing/masking takes place; (2) Time for surface recovery may be shorter; as the epithelium may need less remodelling, reduced post-operative discomfort for the patient; (3) Better final vision, reduced levels of induced Higher Order Aberrations (HOAs), and less haze response
SmartSurfACE treatment (SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany) is a combination of Transepithelial Photo Refractive Keratectomy (Transepithelial PRK), implemented using the Smart Pulse Technology (SPT, SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany), aiming at reducing the residual roughness to enhance the short-term outcomes without compromising stability or long term outcomes.7,8 The ablation profile with SPT is described as a 3 dimensional volume with x/y/z-coordinates based on a curved corneal surface where each position has an equidistant in the 3 dimensional room. Further features incorporated in SPT and SmartSurfACE are discussed later.
Although significant improvements have been achieved with SPT and it's ongoing further development, in simulation and lab environment,6 the clinical impact on immediate and short visual recovery remains to be explored. The aim of this study is to investigate immediate and short-term visual recovery in a large cohort of more than 2000 myopic eyes (with or without astigmatism) treated with SmartSurfACE procedure. In this retrospective case series, the post-operative outcomes were evaluated immediately after the surgery (Day 0), at day 1 and 3 months postoperatively, after myopic SmartSurfACE treatment. Additional aims of this study were to compare the postoperative immediate binocular visual acuity in eyes with moderate myopia (up to −7D preoperatively) to eyes with high myopia (−7D and higher).
MethodsPatientsThis retrospective study was based on a series of patients treated by two surgeons (DTCL, SH), with the SmartSurfACE technique to correct myopia with and without astigmatism, at the Pacific Laser Eye Centre, Vancouver, Canada. Informed consent was obtained from each patient, for both the treatment and use of their de-identified clinical data for publication. The investigation in this form is not subject to Medical Research Involving Human Subjects Act (WMO). The outcomes of performing SmartSurfACE in 2093 consecutive eyes (of 1067 patients) were retrospectively analyzed.
The average age of the patients was 34.9±9.53 years (range 18–70 years). The mean preoperative spherical equivalent was −4.65D±2.53 (−16.13 to −0.13D), with mean preoperative astigmatism −0.85D±0.83 (−8 to 0D). Inclusion criteria for the retrospective chart review were patients older than 18 years, treated with SmartSurfACE treatment, myopic (with or without astigmatism).
Preoperative assessmentA full ophthalmologic examination was performed on all the patients prior to surgery including manifest refraction, cycloplegic refraction and corneal topography (SCHWIND Sirius, SCHWIND eye-tech-solutions GmbH) performed over a diameter of 4.5mm. Corrected distance visual acuity (CDVA) and uncorrected distance visual acuity (UDVA) were assessed with Early Treatment Diabetic Retinopathy Study (ETDRS) charts. The corrected visual acuity was assessed with trial frames and not contact lenses. All the tests were performed monocularly and binocularly.
Surgical procedureAll the treatments were prepared using the SCHWIND Custom Ablation Manager in Aberration-Free mode (SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany). SmartSurfACE treatment was planned for each eye based on the total manifest refraction (sphere and cylinder correction in diopters). The devices used in this study meet the standards of European conformity (Conformité Européene or CE marking) but are not approved by the U.S. Food and Drug Administration (FDA).
For each treatment, the planning software calculated the size of the optimal transition zone, depending on the preoperative refraction and optical treatment zone. Drops of topical anaesthetic were instilled in the upper and lower fornices. A sterile drape covering eye lashes was used to isolate the surgical field. A lid speculum was inserted to allow maximum exposure of the globe.
Accurate alignment of the eye with the laser was achieved with a 1050Hz infrared eye tracker with simultaneous limbus, pupil, and torsion tracking integrated into the laser system and centred on the corneal vertex. The eye tracker had a response time of 1.7ms with a system total latency time of 2.9ms. The ablation profile was centred on the corneal vertex determined by the topography (taking 100% of the pupil offset value),9 which closely approximates the visual axis.10,11 Further, the topographic keratometry readings at 3mm diameter were used for the compensation of the loss of efficiency when ablating the cornea at non-normal incidences. Patients were requested to look at a pulsing green fixation light throughout the ablation. Mitomycin C (MMC) 0.02% was applied for 30s at the conclusion of the ablation, the ocular surface then thoroughly washed with balanced salt solution, a bandage contact lens applied and both topical antibiotics and corticosteroid eye drops instilled.
Patients received topical antibiotic drops QID for 1 week; corticosteroid drops QID tapering off in 1 week and ocular lubricants as needed.
Postoperative evaluationPatients were reviewed immediately after the surgery (Day 0 – within 30min post treatment), at Day 1 and three months post operatively.
Statistical analysisVisual acuity was evaluated in logMAR. The analysis comprised evaluating the change in binocular and monocular visual acuity for all the eyes, preoperatively and at each follow up. The paired Student's t-test were used to evaluate the difference between preoperative and postoperative visual acuity. A p value less than 0.05 was considered statistically significant.
Comparison of low-to-moderate and high myopiaIn addition to the main aims of the study, further analysis was performed to compare eyes with preoperative refraction of up to −7D, to eyes with preoperative refraction equal or higher than −7D, in terms of their achieved binocular visual acuity immediately (at Day 0) after performing SmartSurfACE procedure.
ResultsOut of 2093 eyes, 100% eyes (n=2093) completed Day 0 follow up, 96% eyes (n=2014) completed Day 1 follow up, and 48% eyes (n=1001) completed 3-month follow up.
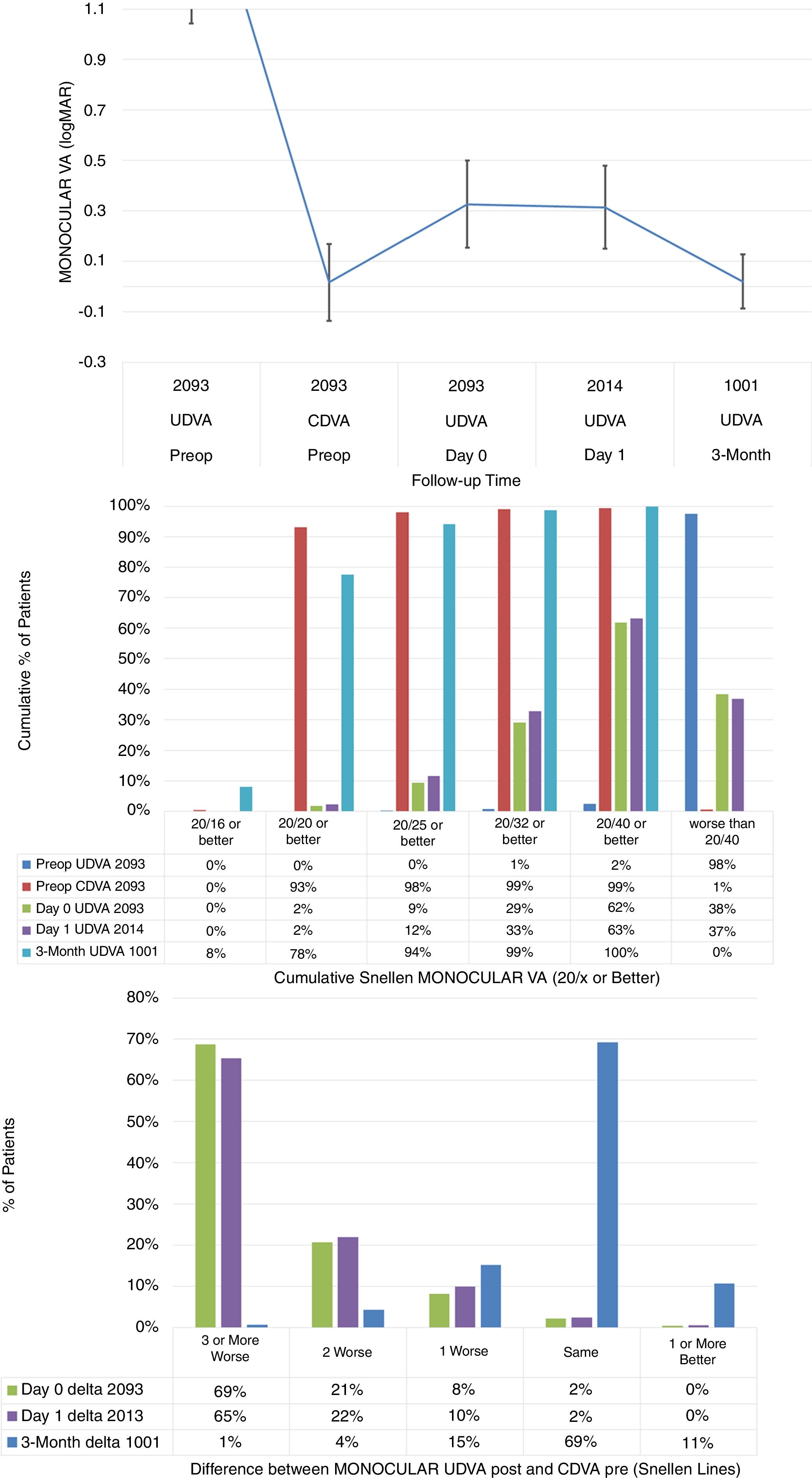
Monocular visual acuityThe stability in monocular visual acuity, cumulative Snellen Monocular Visual Acuity, and the difference between the monocular postoperative UDVA and preoperative CDVA are presented in Fig. 1 (Fig. 1: Top, centre and bottom respectively).
The stability in monocular visual acuity, cumulative Snellen Monocular Visual Acuity, and the difference between the monocular postoperative Uncorrected Distance Visual Acuity (UDVA) and preoperative Corrected Distance Visual Acuity (CDVA) (Top, centre and bottom respectively) in 2093 eyes (of 1067 patients), at day 0, day 1 (immediately after) and 3-months (short-term) after performing SmartSurfACE treatment.
Immediately after the surgery or on the next day, treated eyes achieved average UDVA 20/41±8 (Fig. 1, left). Achieved UDVA in different follow up visits were compared statistically. For the left eyes, the average UDVA improved from 0.34±0.20logMAR immediately after the surgery, to 0.32±0.16logMAR at day 1 postoperatively. This improvement of 0.3±1.9 Snellen lines was statistically significant (p<0.0001), with the third percentile of the gained lines being 0.1lines (above zero and significant). For the right eyes, the average UDVA remained stable changing from 0.30±0.15logMAR immediately after the surgery, to 0.30±0.16logMAR at day 1 postoperatively, not changing significantly (p=.5). UDVA improved significantly from Day 1 to 3-months follow up (p<0.0001 for both OS and OD); by 3-months postoperatively, the treated eyes could see on average UDVA 20/21±5 (equal to preoperative CDVA 20/21±8).
Immediately after the surgery or the next day, more than 60% of the eyes could see UDVA 20/40 or better (Fig. 1, middle). By 3-months postoperatively, 94% of the eyes could see UDVA 20/25 or better. In terms of the change in Snellen lines, immediately after the surgery or the next day, more than 30% of the eyes could see UDVA within 2 lines of preoperative CDVA (Fig. 1, right). By 3-months postoperatively, 95% of the eyes could see UDVA within a line of preoperative CDVA.
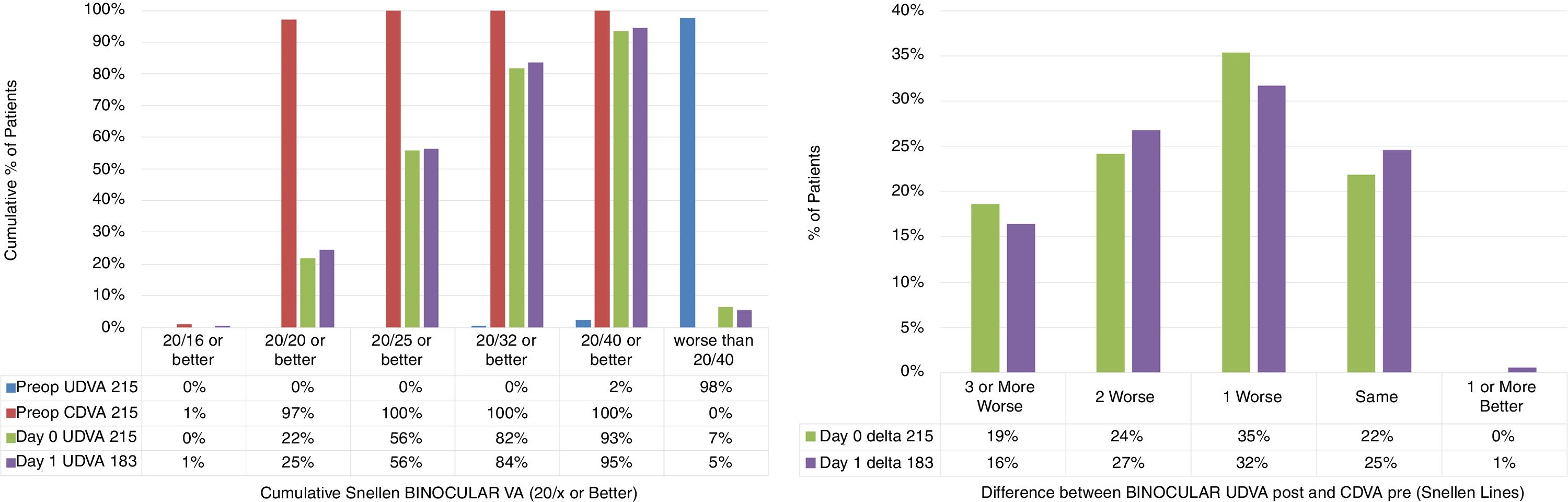
Binocular visual acuityThe cumulative Snellen Binocular Visual Acuity and the difference between the Binocular postoperative UDVA and preoperative CDVA are presented in Fig. 2 (Fig. 2: Left and right respectively).
The cumulative Snellen Binocular Visual Acuity and the difference between the Binocular postoperative Uncorrected Distance Visual Acuity (UDVA) and preoperative Corrected Distance Visual Acuity (CDVA) (Left and right respectively) in 215 patients, at day 0, and day 1 (immediately after) after performing SmartSurfACE treatment.
Immediately after the surgery as well as the next day, more than 80% of the patients could see UDVA 20/32 or better (Fig. 2: Left). In terms of the change in Snellen lines, immediately after the surgery or the next day, more than 50% of the patients could see UDVA within a line of preoperative CDVA (Fig. 2: Right).
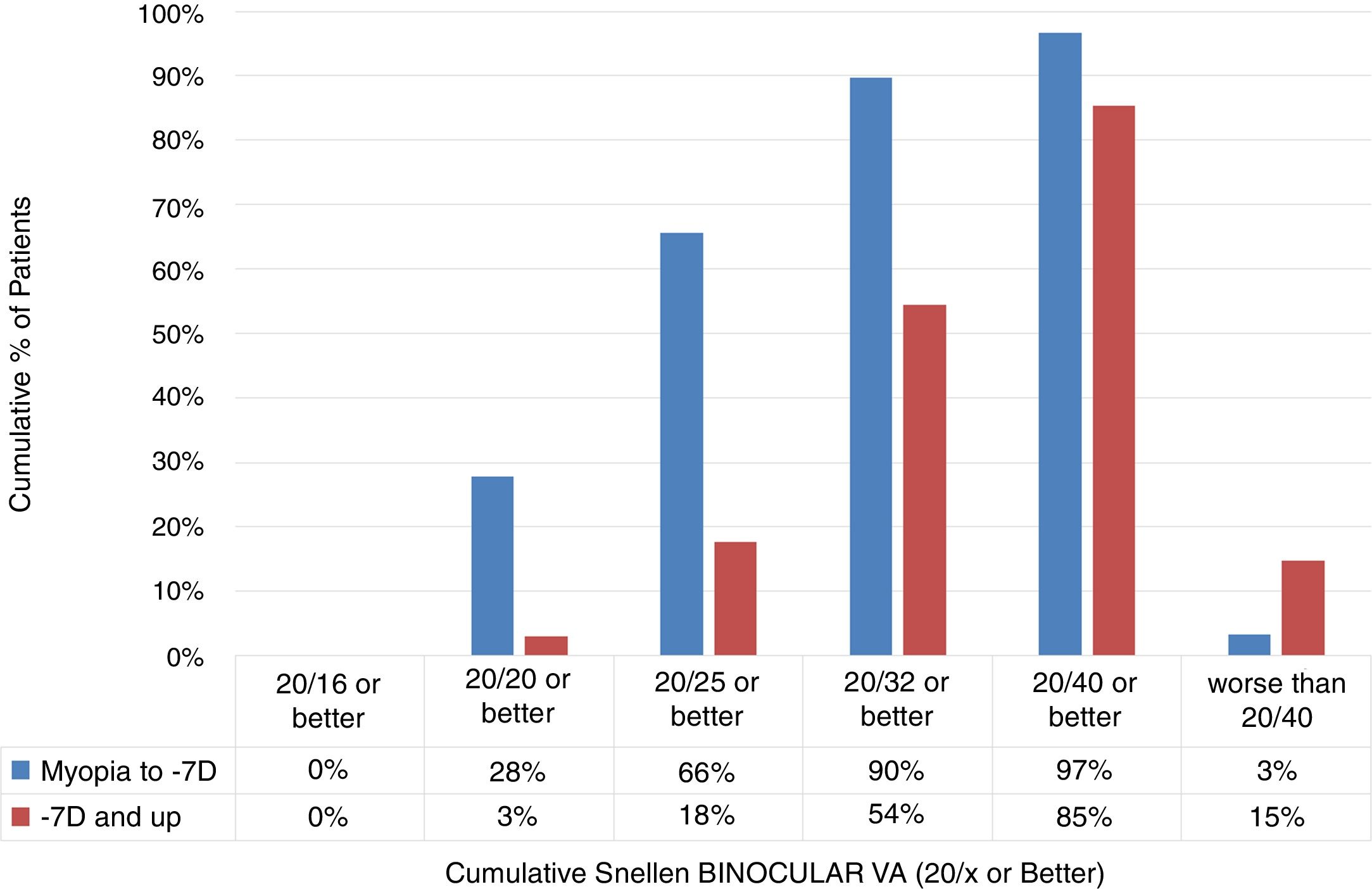
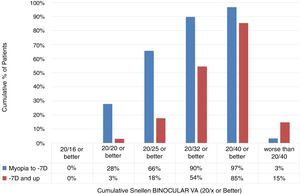
Comparison of low-to-moderate and high myopiaThe cumulative Snellen Binocular Visual Acuity immediately (at Day 0) after performing SmartSurfACE procedure in the two groups, is presented in Fig. 3. One hundred fifty one patients were analyzed in the Low-to-Moderate myopia group while 34 patients were analyzed in the high myopia group. Immediately after the surgery, more than 90% of the patients in the low-to-moderate Myopia group (up to −7D) could see UDVA 20/32 or better, whereas in the high myopia group (−7D and up), 54% of the patients could see UDVA 20/32 or better (Fig. 3).
DiscussionCorneal excimer laser ablations result in increases in surface roughness, surface contraction, and stromal morphologic change. Such surface changes are an important factor in any laser refractive correction procedure, where smoother ablated surfaces show more efficient refractive correction, induce less scatter postoperatively, and present a more regular optical surface for a faster healing.7,8 Furthermore, postoperatively seen stromal roughness can be simply expressed as sequences of stromal peaks and troughs. In the natural healing process of the cornea, the epithelium would need to fill up the troughs to the level of the peaks to eliminate roughness and regularize the corneal surface.
The aim of this study was to clinically evaluate the impact of Smart Pulse Technology in combination with Transepithelial PRK (SmartSurfACE) on immediate and short visual recovery. Whether the improvements imparted through ablation smoothness observed in the laboratory environment translate into improvements in the real world clinical outcomes highly depends on the threshold of surface roughness affecting the vision in human eyes. The smoothness achieved with SmartSurfACE occurs at the stromal level of the cornea. Hence, the maximum effect of the smoothness potentially imparted through the procedure, will be observable immediately after the ablation, before epithelialization has begun. This effect will progressively decrease as the epithelium regrows leading to a masking effect, further vanishing as the epithelium remodelling is completed. Therefore, in order to highlight these effects in postoperative outcomes, 1-day postoperative visual acuity was analyzed in this study, instead of one-week follow up results.
The inception of SPT dates back from AMARIS origins, but its realization took finally place during 2014.12–14 Some commercially available laser systems for laser vision correction15 use the deconvolution method to decompose the ablation volume into pulses.16–18 Previous generations of the AMARIS laser system used a simple heuristic search, without local or global optimizations. With SPT, the simple heuristic search was expanded to a global optimization problem, which finds a balance between minimum residual roughness and maximum ablation precision.6
The impact of this ablation algorithm was seen in terms of the quick recovery in visual acuity after the SmartSurfACE treatments in our cohort; with the achieved immediate average UDVA=20/41±8, improving further at 3-months postoperatively to an average UDVA of 20/21±5 (equal to preoperative CDVA 20/21±8). In our cohort, immediately after the surgery, the eyes in low-to-moderate Myopia group (up to −7D) performed slightly better than the high myopia group. The sub-analysis comparing the visual recovery in eyes with low-moderate myopia (up to −7D) versus eyes with high myopia (>−7D), included only 151 patients with low-moderate myopia (302 eyes) compared with 34 patients with high myopia (68 eyes), so the total number of eyes included in this sub-analysis was only 370. In this retrospective study, binocular vision was not measured systematically for all eyes at all follow-ups (while monocular VA was), and for that reason we can only provide binocular values of that “subgroup”, for which there was no systematic inclusion criteria bias.
Similar studies have been published in the past to evaluate the immediate and short-term visual acuity in other refractive procedures like thin-flap femto-LASIK, LASIK and PRK. Durrie DS et al.19 investigated the speed of visual recovery following myopic thin-flap LASIK with a femtosecond laser. In this pilot study, they prospectively evaluated 20 eyes from 10 patients who underwent bilateral simultaneous LASIK. The Femto LDV Crystal Line femtosecond laser (Ziemer Ophthalmic Systems AG) was used to create a circular flap of 9.0-mm diameter and 110-μm thickness followed by photoablation with the Allegretto Wave Eye-Q (WaveLight AG) excimer laser. In their cohort, 100% of eyes achieved monocular UDVA of 20/40 at 1h and 20/25 at 4h. For binocular UDVA, all patients achieved 20/32 by 30min and 20/20 by 4h. In comparison, in our cohort where surface ablation was performed, 62% eyes achieved monocular UDVA 20/40 or better immediately after the surgery, while 82% patients achieved binocular UDVA 20/25 or better immediately after the surgery.
Hersh PS et al.20 published results of a randomized clinical trial of PRK and LASIK, where a total of 220 eyes of 220 patients entered the study cohort: 105 randomized to PRK and 115 to LASIK. The mean preoperative manifest refraction spherical equivalent was −9.23 diopters (D) in the PRK group and −9.30 D in the LASIK group. They reported that one day after surgery, 0 (0.0%) and 3 (4.5%) eyes in the PRK group saw 20/20 and 20/40 or better UDVA, respectively, while 7 (10%) and 48 (68.6%) eyes in the LASIK group saw 20/20 and 20/40 or better, respectively. After PRK, eight eyes (11.8%) had a decrease in CDVA of two Snellen lines or more; after LASIK, two eyes (3.2%) had a decrease of two lines or more (odds ratio=3.89 for risk of loss of spectacle-corrected visual acuity for PRK vs. LASIK, 95% CI=0.71–21.30). In comparison, in our cohort, 2% and 63% eyes saw 20/20 and 20/40 or better UDVA, at 1-day follow up respectively. Walker and Wilson21 also compared uncorrected visual acuity and refractive error in patients undergoing PRK and LASIK between 1 week and 6 months after surgery. They concluded that uncorrected visual acuity 1 week after surgery is significantly better in eyes undergoing LASIK than in eyes undergoing PRK and the difference does not relate to refractive error, which was similar between the two groups, but to differences in healing of the epithelium. They found no differences between the groups 1 month after surgery. There is a lack of published results on short term visual recovery in LASIK to allow a direct comparison with Transepithelial PRK. These smaller studies may not be usefully comparable to the very large cohort in the present study (20 eyes of 10 patients,19 105+115 eyes of 220 patients20 vs. >2000 eyes of >1000 patients).
In our cohort, we used aberration neutral profiles using the Aberration-Free mode during treatment planning (SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany). The clinical success of aspheric ablation profiles has been well published in the literature. Arbelaez et al.22 evaluated the clinical outcomes of aspheric corneal wavefront guided ablation profiles in LASIK treatments. In general, they reported improvements in postoperative UDVA and CDVA (p<0.001). They concluded that apart from the risk of additional ablation of corneal tissue, systematic wavefront-customized corneal ablation can be considered as a safe and beneficial method. Centration reference during ablation is another critical aspect for successful postoperative outcomes.23 It has been theoretically postulated and shown that aberration-free profiles be centred on the corneal apex, whereas customized treatments centred according to the diagnosis reference. The main high order aberration effects (coma and spherical aberration) come from the edge effect, which is the strong local curvature change from the optical zone to transition zone and from transition zone to non-treated cornea.24 In this study, asymmetric offset was used; this approach combines the higher order aberrations (HOA) referred to the pupil centre (line-of-sight) with manifest refraction values referred to the corneal vertex (visual axis).9 Clinically it has been shown that in myopic eyes with moderate to large pupillary offset, corneal vertex centred treatments perform better in terms of induced ocular aberrations and asphericity.11
There are several limitations of our study. Out of the 2093 eyes, 48% eyes (n=1001) completed 3-month follow up. This drop-out rate can be regarded as high, however, due to the large patient cohort (∼2000 eyes), in the three month follow, ∼1000 eyes could be analyzed. In order to reduce this risk of bias, only those eyes that completed all the postoperative visits (day 0, day 1 and 3 months) could have been included. This problem is well known in retrospective studies, and for that reason we performed paired analyses, i.e. the 3M analyses only compare to the paired preop eyes. For the 1day follow up we have 96% follow-up. The 3M analyses were only a “sanity check” for the cohort, to see that the 3M outcomes were “normal”. With such large numbers, one follows the intuition that increasing the sample size may result in statistical significances (through reduced standard error), but the mean values and standard deviations (spread of the data) will not change largely.
Due to the large range of myopia treated in our cohort, emmetropia was not the intended target of the surgery in some eyes. The main evaluation criterion in this study was uncorrected visual acuity and it could be argued that only eyes treated for emmetropia be analyzed. Having any other target besides emmetropia would reduce the UDVA. Therefore, our results showing a good short term visual recovery reinforces the benefits of the treatment. The influence of the small number of eyes having a non-emmetropic target would be minimal due to the large cohort size where majority of the eyes underwent treatment with emmetropia as a target.
SmartSurfACE treatment is a combination of Transepithelial PRK, implemented using the Smart Pulse Technology. An interesting question to pursue is whether the addition of SPT provides rapid visual recovery over transepithelial PRK. We acknowledge that this comparison (SmartSurfACE vs. transepithelial PRK) will add value to the results presented in this study. However, the number of cases with Transepithelial PRK in our practice were too small to be comparable to the SmartSurfACE cases, causing imbalance in cohort sizes and a biased comparison. Our impression was that immediate post-operative outcomes with Transepithelial PRK in our practice were not ideal, thus inspiring us to develop SPT technology in order to improve upon Transepithelial PRK outcomes for a faster visual recovery. Upon availability and due to the initial positive experience with SmartSurfACE, it became the treatment of choice at our practice for surface ablation procedures.
In order to analyze the smoothness of the SmartSurfACE, that is a combination of transepithelial PRK implemented with the Smart Pulse Technology, we evaluated the immediate visual recovery at day 0 and day 1 postop, because the maximum effect of the smoothness will be observable immediately after ablation, before epithelialization has begun. However, the therapeutic contact lens offers a smooth surface that could mask or compensate some of the irregularity of the ablated stroma, thus improving by itself the visual acuity of the patient. The best way to analyze if the Smart Pulse Technology improves the immediate postoperative visual results after transepithelial PRK should be a comparative eye-paired study, in which one eye should be treated only with a transepithelial PRK and the other eye of the same patient treated with the SmartSurfACE.
Although, for a sample size of sufficient power, one could avoid the fellow-eye comparison. Such comparison has been performed for a moderate sample size by Aslanides and Kymionis7 and Vinciguerra et al. to some extent.8
In addition, it would be advisable to compare not only the immediate visual recovery, but also the re-epithelialization rate after both procedures, in order to analyze if the SmartSurfACE offers the advantage of a reduced epithelial healing by improving the stromal roughness. This has been addressed by Aslanides and Kymionis.7
It should be very interesting to analyze if the addition of a Smart Pulse Technology offers the advantage to induce lower HOAs by reducing the roughness of the stromal surface, as compared to a transepithelial PRK alone, using the same excimer laser and the same ablation profile. We think focusing this work on short term visual acuity on a large scale population is a simple yet powerful topic, serving the demands of the patients (they want to regain UDVA as sonn as possible regardless of the applied clinical technique).
In conclusion, in our large cohort of eyes, immediate and short-term visual recovery after SmartSurfACE was rapid, providing functional binocular UDVA immediately after the surgery. We postulate that it is the advanced myopic ablation pattern of the SPT that results in a smoother ablation and thus a more rapid visual recovery for patients.
Conflicts of interestThe authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article. Shwetabh Verma and Samuel Arba Mosquera are employees at SCHWIND eye-tech-solutions, Kleinostheim, Germany. David TC Lin is a consultant to SCHWIND eye-tech-solutions, Kleinostheim, Germany.