To determine the factors associated with amblyopia in a referral clinical population.
MethodsIn this cross-sectional study, 164 subjects who were referred to an amblyopia clinic were enrolled and divided into two groups: refractive amblyopia group and refractive non-amblyopia group. Visual acuity, refractive measurements, and information on birth parameter and delivery mode were compared between both groups.
ResultsWe included 164 children (91 children in the non-amblyopic group and 73 children in the amblyopic group) aged 5–10 years. 50.6% of children with amblyopia had anisometropia, defined as a difference among eyes in spherical equivalent of 1.00D or more. The regression analysis revealed that amblyopia was strongly associated with hyperopia ≥2.00D (odds ratio, 10.0; 95% CI, 3.27–30.58), anisometropia ≥1.00D (odds ratio, 7.78; 95% CI, 3.64–16.61), astigmatism ≥1.00D (odds ratio, 5.23; 95% CI, 2.48–11.02), and myopia ≥−2D (odds ratio, 6.96; 95% CI, 1.9–25.28). There were also significant associations of amblyopia with low birth weight (≤2500g), preterm birth (≤37 weeks), and dystocia (all P<0.001).
ConclusionPrematurity, low birth weight, and dystocia as well as refractive errors were associated with amblyopia in our select patient population.
Determinar los factores asociados a la ambliopía en una población clínica infantil.
MétodosEn este estudio transversal, 164 pacientes remitidos a una clínica fueron incluidos en el estudio y divididos en 2 grupos: el grupo de ambliopía y el grupo refractivo no ambliope. Se comparó entre los dos grupos la agudeza visual, los datos refractivas y la información sobre parámetros de nacimiento y modo de parto.
ResultadosIncluimos a 164 niños (91 niños en el grupo no ambliópico y 73 niños en el grupo ambliópico), con edades comprendidas entre 5 y 10 años. El 50,6% de los niños con ambliopía tenían anisometropía, definida como la diferencia en equivalente esférico entre ojos de 1.00 D o más. En el análisis de regresión, la ambliopía estaba fuertemente asociada a hipermetropía ≥ 2,00 D (odds ratio, 10,0; 95% CI, 3,27–30,58), anisometropía ≥1,00 D (odds ratio, 7,78; 95% CI, 3,64– 16,61), astigmatismo ≥1,00 D (odds ratio, 5,23; 95% CI, 2,48–11,02), y miopía ≥ -2 D (odds ratio, 6,96; 95% CI, 1,9-25,28). También se produjeron asociaciones significativas de la ambliopía con el bajo peso al nacer (≤2500g), nacimiento antes de término (≤37 semanas), y distocia (todos P<0,001).
ConclusiónLa prematuridad, el bajo peso al nacer y la distocia, además de los errores refractivos, estaban asociados a la ambliopía en nuestra población de pacientes seleccionados.
A few studies have shown that visual impairment in young children may not only lead to a reduction in quality of their lives but will also affect them in the social setting.1 Refractive error has been the cause of reduced vision in 56–90% of eyes in two large population based studies in Chile and China.2,3 Refractive errors can be corrected with glasses but visual impairment associated with amblyopia may need additional therapeutic intervention. Failure to adequately address amblyopia places the subjects at risk of permanent visual loss.4 Proper clinical examination as well as various vision screening tests are essential to adequately identify children with amblyogenic risk factors in order to set in place appropriate treatment modalities.5
Despite the recognized importance of correcting refractive anomalies in children, available data are incomplete concerning the prevalence of refractive error in different geographical areas and its variation with sex and race. According to Tehran Eye Study, the prevalence of myopia and hyperopia in children aged 5 to 15 years was 7.2% and 76.2%, respectively.6 This study was designed to collect the data from a referral amblyopia clinic in Qazvin province (Iran) and to study its associated refractive errors including visual acuity (VA) and spherical equivalent (SE) distribution.
In addition, previous studies have investigated the various birth parameters associated with refractive error and amblyopia. Prevalence of strabismus7 and amblyopia8,9 may be higher in preterm children without retinopathy of prematurity than in age-matched, normally delivered children. Risk of refractive errors is also higher in preterm infants than in infants born at term.10 Other studies also found that prematurely born infants run an increased risk of having hyperopia, myopia, and anisometropia.10–13 Overall, refractive errors are four times more common in those born preterm than those born at term.12 In this study, we also studied the association of birth parameters (prematurity, low birth weight, and dystocia) with amblyopia.
MethodsParticipantsQazvin preschool and school vision screening program was part of the Iran nationwide vision-screening program that was conducted in 2000. Nationwide school-based screening program had been consisting of two-step process, beginning with public healthcare centers, and then referring patients for comprehensive professional examinations in eye clinics. Healthcare centers screened 12,300 children using the tumbling E-visual acuity eye chart. On the basis of this vision testing, children who had VA of less than 20/30 at preschool years, VA of less than 20/25 at school years in either eye or who had two or more lines of difference between the eyes were referred to the single eye clinic (Qazvin University of Medical Science). Other referral criteria were history of suspected ocular misalignment and other ocular diseases. A total 304 children were referred from 2006 to 2009 and all were examined. Examination procedures were approved by the University of Qazvin ethics committee, and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all parents before examinations. At the eye clinic, routine ophthalmological evaluation was performed by a technician and confirmed by one of the authors (M.M.). Uncorrected monocular VA was measured using the single-surround E optotypes read at 6m and cycloplegic refraction was performed on the day of visit. If the VA was not 20/20, the last line read by the subject was retested. If this level was again failed, the test was then stopped. If the level was passed, the test continued with smaller levels until the particular level was failed. The final VA was defined as the best VA line passed by the patient. Children were also examined wearing their own spectacles if they were brought to the examinations. Automatic cycloplegic refraction was performed on all children after adequate cycloplegia achieved using two drops of cyclopentolate 1% (Sina Daru, Tehran, Iran). Refraction was measured 25min after the last cycloplegic eye drops instilled in the eye using a table-mounted autorefractor (Canon Autorefractor RK-F1; Canon, Tokyo, Japan). If autorefraction measurements failed after multiple attempts, streak retinoscopy in a dimly lit room was performed. Subjects’ objective cyclorefraction was refined with subjective refraction in all children one week later and best-corrected VA was measured. If a child was already wearing glasses, a new prescription was not given as long as both the spherical equivalent and cylinder were within 0.50D of subjective refraction. Uncorrected and best-corrected VA were converted to logarithm of minimum angle of resolution (logMAR) for statistic analysis. Alternate cover–uncover test at distant and near fixation, ocular motility testing, and examination of the ocular media and fundus were performed. Alternate cover–uncover with prism was performed with full optical correction if there was a significant refractive error. Subjects with less than 10 prism diopter of esodeviation were known to have microtropia and were excluded. As a result, we did not perform sensory testing on these patients. Of 304 children, 40 children had normal visual acuity and examination and were excluded. One hundred children were also excluded because of cataract (n=5), strabismus (n=65), coexisting fundus or anterior segment abnormalities (n=30). Thus, 164 children were included in this cross-sectional study and divided into two groups: the refractive amblyopic group and the refractive non-amblyopic group.
Refractive amblyopic group: Refractive amblyopia was defined using modified Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) criteria for refractive amblyopia (excluding strabismic and deprivational amblyopia criteria), and divided into unilateral and bilateral subtypes.14 Unilateral refractive amblyopia was defined as a 2-line difference in best corrected VA between the two eyes consistent with the presence of anisometropia (≥1.00 diopters [D] SE anisohyperopia, ≥3.00D SE anisomyopia, or ≥1.50D anisoastigmatism). Bilateral refractive amblyopia was defined as bilaterally reduced best corrected VA (less than 20/30) with bilateral ametropia quitar (≥4.00D SE hyperopia, ≥6.00D SE myopia, or ≥2.50D astigmatism).
Refractive non-amblyopic group: Refractive error without amblyopia and therefore best corrected VA of more than 20/25 in both eyes after correction of refractive error.
Refractive errors definition: Myopia was defined as SE (sphere+½ cylinder) of at least – 0.50D, hyperopia as SE ≥2.00D, astigmatism as cylinder power ≥1.00D, and anisometropia as an SE difference ≥1.00D between the two eyes. In case of myopia, hyperopia, or astigmatism, the data from the most ametropic eye was presented.
Delivery mode and birth parametersDelivery mode divided to: (1) natural labor (normal vaginal delivery), (2) cesarean birth, (3) dystocia of normal labor. Dystocia of normal labor was defined as abnormal labor pattern at any three stages of labor.15 Low birth weight was defined as birth weight ≤2500g and premature birth was defined as gestational age ≤36 week. These data were obtained from past medical records of mothers and confirmed by a pediatrician.
Statistical analysisAll statistical analyses were performed using SPSS for Windows Version 17.0 (SPSS, Inc., Chicago, IL, USA). The paired t-test and Chi-square tests were used to compare means and proportions of categorical factors in the amblyopic and non-amblyopic groups with statistical significance at P<0.05. Histograms with normal curve were used to confirm that our sample data followed a normal distribution. We also carried out a logistic regression analysis with amblyopia as the dichotomized outcome and anisometropia, hyperopia, myopia, anisometropia, and birth parameters as independent predictors to determine the strength of association of each factor with amblyopia. In logistic regression, all variables were entered in one single step (“Enter” default in SPSS software). Odds ratios (OR), and 95% confidence intervals (CI) were reported.
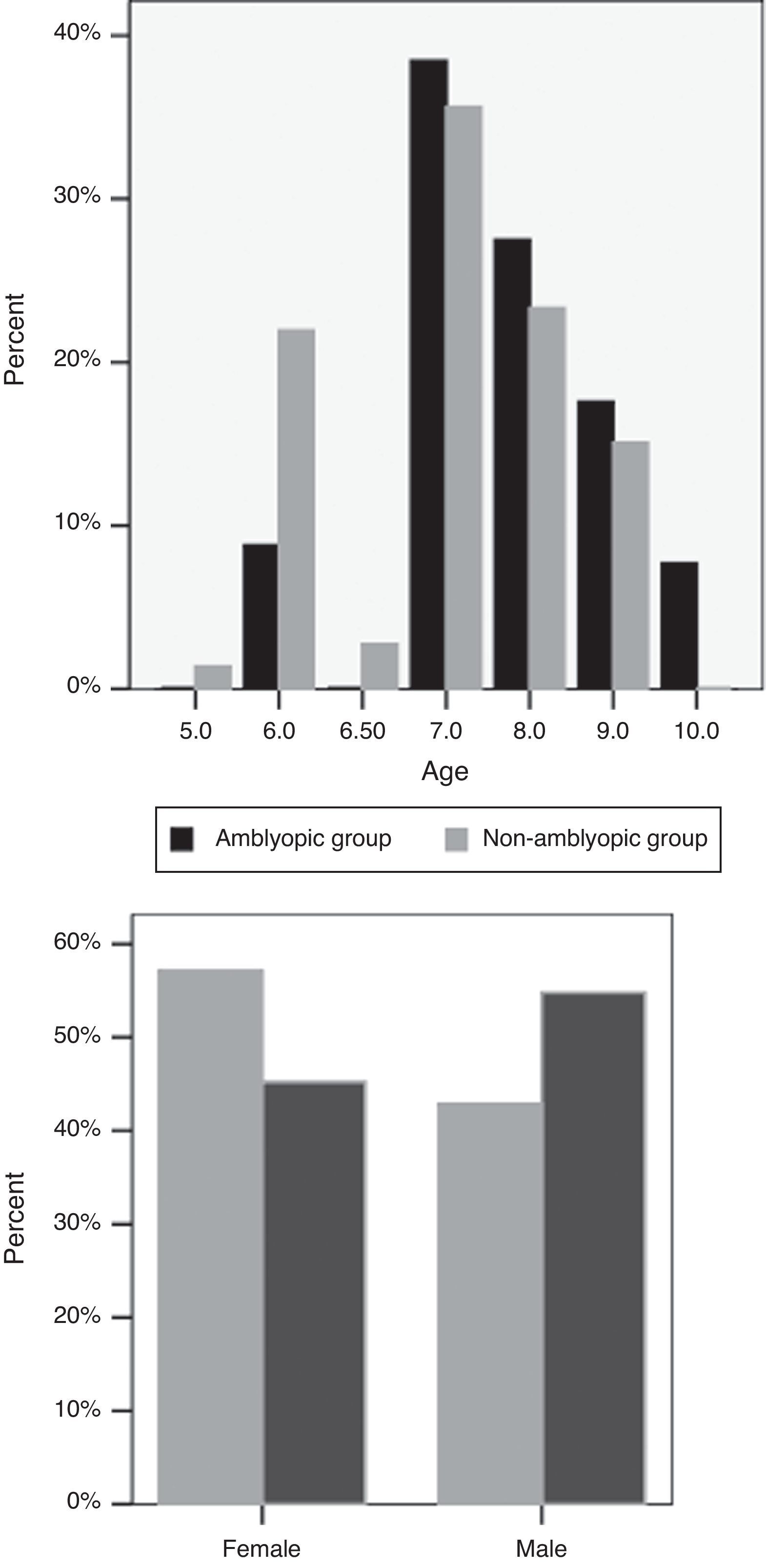
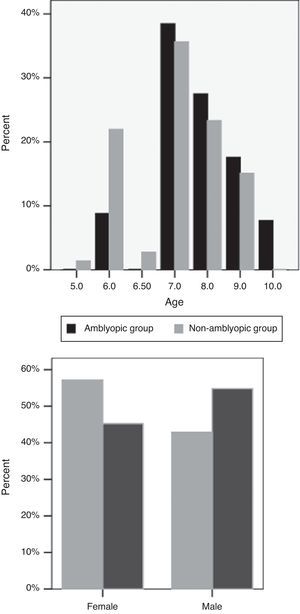
ResultsOf the 164 participants in this study, 79 (48.2%) were male. The mean age of the subjects was 7.54±1.08 (range, 5–10 years). Mean sphere and cylinder errors were 0.11±1.30D (range, −3.5 to 4) and −.29±0.91D (range, −3 to 2.5), respectively. There were 91 children in the non-amblyopic group and 73 children in the amblyopic group. The mean age of the subjects in the non-amblyopic group and the amblyopic group were 7.76±1.08 years and 7.27±1.02 years, respectively (P=0.62) (Fig. 1). 42.9% of subjects in the non-amblyopic group and 54.8% of subjects in the amblyopic group were male (P=0.12, Chi square) (Fig. 1). Mean sphere and cylinder of the subjects in the non-amblyopic group were −0.25±0.89D and −0.08±0.40D. Corresponding measures were 0.57±1.57D and −0.55±1.24D in the amblyopic group. There were significant differences in sphere and cylinder errors between the two groups (P<0.001, P<0.01).
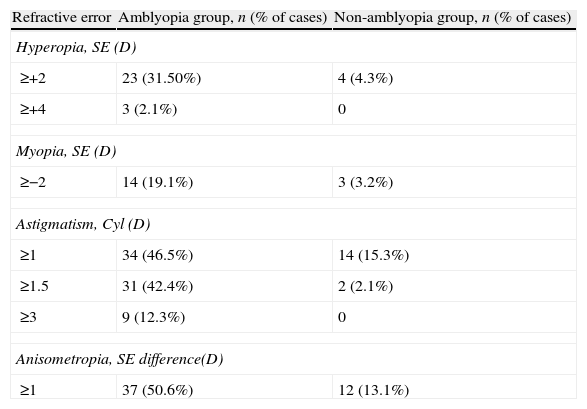
Thirty-seven (50%) subjects in the amblyopia group had bilateral amblyopia. Of the 73 children with amblyopia, 37 (50.6%) had anisometropia quitar esto, ya esta definido antes. Around half (34 cases, 46%) of the amblyopic children had astigmatism ≥1.00D, of whom 20 (27.4%) had astigmatic errors of 2.5D to 3.00D. Nine subjects in amblyopia group had astigmatism ≥3D, 3 cases had SE hyperopia ≥4.00D and 4 cases had SE myopia ≥6.00D. Table 1 shows the refractive distribution of cases with and without amblyopia.
Refractive distribution of the amblyopic and non-amblyopic children (Cyl: cylinder power; D: diopters; SE: spherical equivalent).
| Refractive error | Amblyopia group, n (% of cases) | Non-amblyopia group, n (% of cases) |
| Hyperopia, SE (D) | ||
| ≥+2 | 23 (31.50%) | 4 (4.3%) |
| ≥+4 | 3 (2.1%) | 0 |
| Myopia, SE (D) | ||
| ≥−2 | 14 (19.1%) | 3 (3.2%) |
| Astigmatism, Cyl (D) | ||
| ≥1 | 34 (46.5%) | 14 (15.3%) |
| ≥1.5 | 31 (42.4%) | 2 (2.1%) |
| ≥3 | 9 (12.3%) | 0 |
| Anisometropia, SE difference(D) | ||
| ≥1 | 37 (50.6%) | 12 (13.1%) |
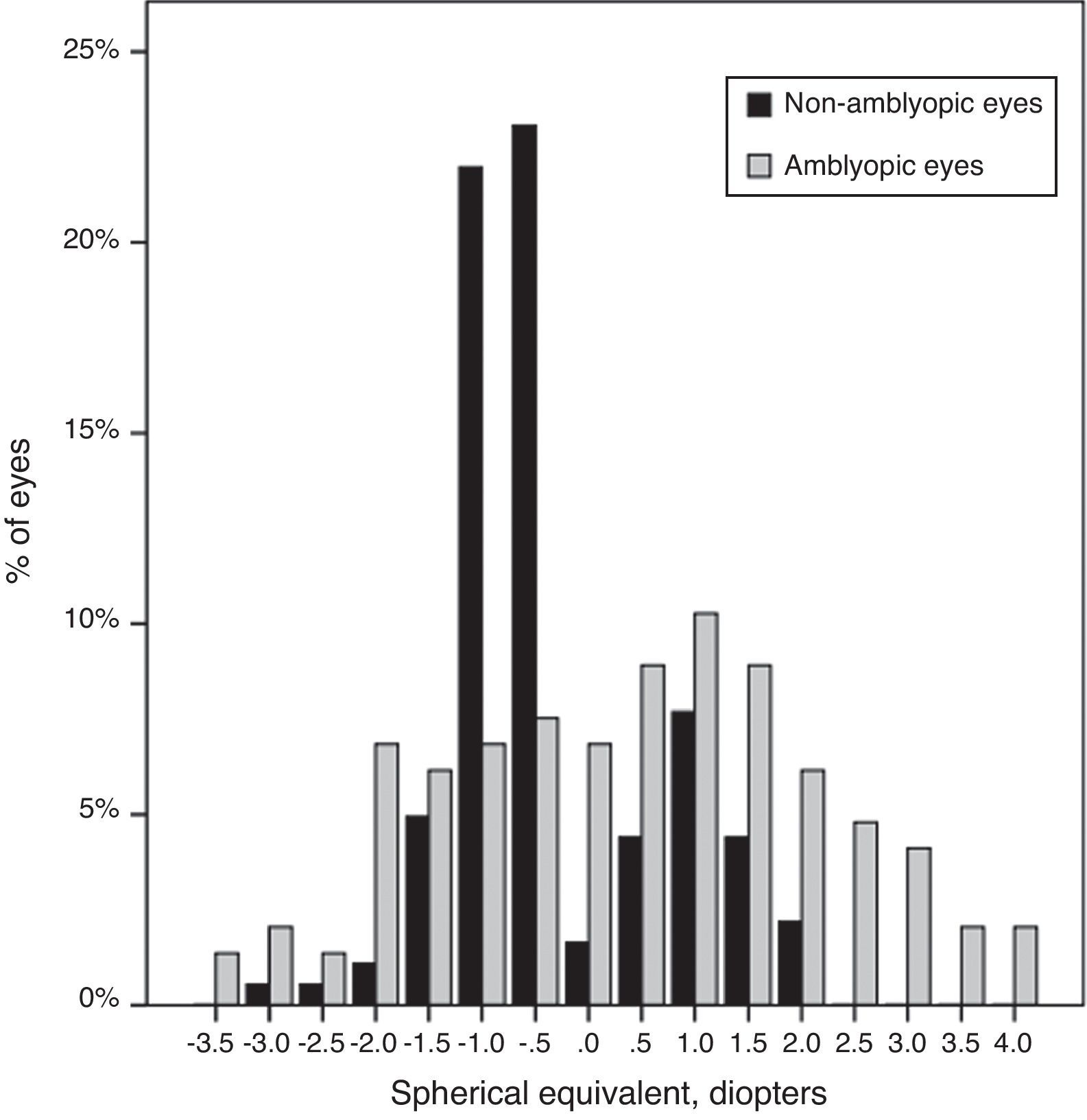
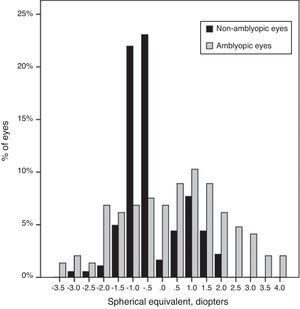
Mean spherical equivalent of the non-amblyopic group was −0.30±0.89D, compared to 0.29±1.6D in the amblyopic group (P=0.00). The SE showed a range of ±4.00D (Fig. 2).
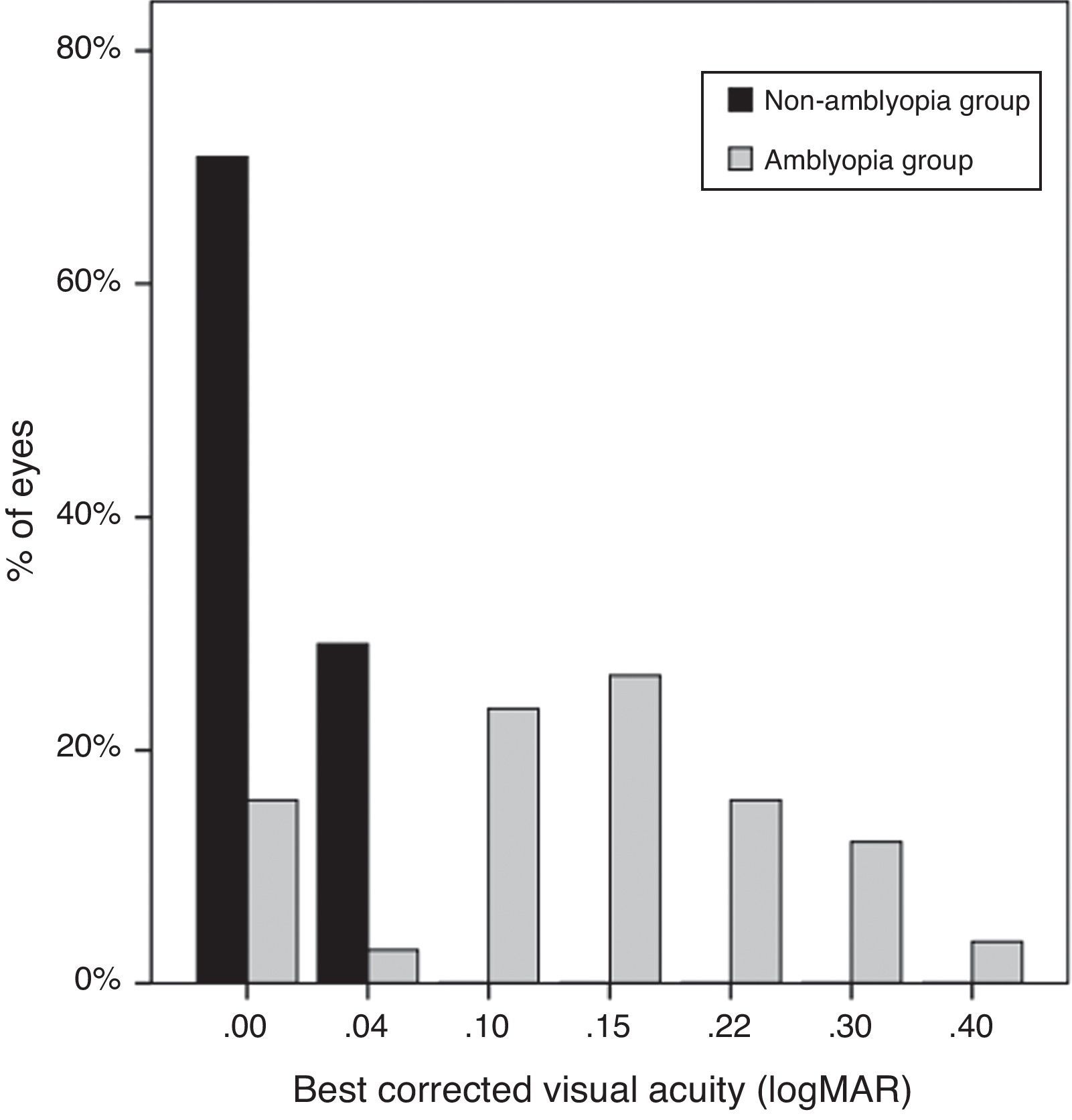
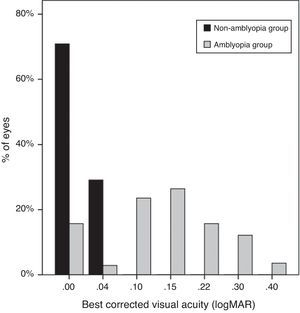
Uncorrected VA of subjects was 0.23±0.13logMAR in the non-amblyopic group, and 0.39±0.26logMAR in the amblyopic group (P=0.00). The mean best-corrected VA of the amblyopic eyes was 0.14±0.10logMAR (20/50), significantly worse than the mean VA (20/20, 0.01±0.01logMAR) of the non-amblyopic eyes (P=0.00) (Fig. 3).
Multivariable logistic regression identified hyperopia, myopia, astigmatism, anisometropia, prematurity, low birth weight, and dystocia as factors significantly associated with amblyopia (P=0.001, P=0.01, P=0.001, P=0.001, P=0.007, P=0.006, and P=0.04, respectively). Amblyopia in this sample was strongly associated with hyperopia ≥2.00D (odds ratio, 10.0; 95% CI, 3.27–30.58), anisometropia ≥1.00D (odds ratio, 7.78; 95% CI, 3.64–16.61), astigmatism ≥1.00D (odds ratio, 5.23; 95% CI, 2.48–11.02), and myopia ≥−2D (odds ratio, 6.96; 95% CI, 1.9–25.28). We found no association of amblyopia with gender (odds ratio, 1.61; 95% CI, 0.86–3.0).
Among the 73 children with amblyopia, nine cases had cesarean birth (12.3%), 10 cases had dystocia of normal labor (13.7%), and 54 cases had natural labor (73.9%). In the non-amblyopia group, 14 cases had cesarean birth (15.3%), 3 cases had dystocia of normal labor (3.2%), and 74 cases had natural labor (81.3%). The proportion of dystocia of normal labor was significantly different between the two groups (P=0.01, Chi-square). Amblyopia in this sample was strongly associated with dystocia (odds ratio, 5.02; 95% CI, 1.33–18.98). We found no associations of amblyopia with cesarean birth (odds ratio, 1.29; 95% CI, 0.52–3.18). Children born at less than 37 weeks’ gestation (≤36 week) had a 7-fold greater risk of having amblyopia (odds ratio, 7.11; 95% CI, 2.28–22.14); 24% of children with amblyopia were born premature compared with 4.4% of children without amblyopia (P=0.000, Chi square). Those with birth weights less than 2500g were almost 6 times more likely to have amblyopia at the time of examination (odds ratio, 6.49; 95% CI, 2.29–18.32).
DiscussionSeveral population-based studies have been performed to determine the prevalence of amblyopia and its associated factors. Previous estimates of amblyopia's prevalence have been at 0.2% in a population-based sample of Iranian residents in Tehran.16 In this study, we collected data from a referral clinic in Qazvin city, which is in close proximity to Tehran.
Amblyopia screening in Qazvin province is based on visual acuity screening in children more than 5 years old. If visual acuity abnormalities were found, the subjects were then referred to the eye clinic for a complete ophthalmological examination. It has been found that repeated preschool vision-screening reduced the prevalence of subsequent (school-aged) amblyopia by 1% compared with a onetime screening.17 In this referral center, half of the amblyopic children had anisometropia ≥1.00D which was the major refractive cause of amblyopia. The second major refractive cause of amblyopia was astigmatism of 2.5–3D which led to the development of bilateral amblyopia. One cohort study also has showed that anisometropia is a common cause of amblyopia, being present as the only identifiable amblyogenic factor in 37% of cases.18 The strongest associations of amblyopia were hyperopia ≥2.00D (odds ratio, 10.0) and anisometropia ≥1.00D (odds ratio, 7.78) in our study. These associations with amblyopia were all statistically significant. Hyperopia ≥2.00D was only seen in 4% of our non-amblyopic group, compared to 31% in the amblyopia group. When mild hyperopia of 2.00D is not amblyogenic per se, we noted that hyperopia had no relevance in 10 children with amblyopia. These 10 children also had anisometropia ≥2.00D which explained the development of amblyopia. In addition, it has been reported that hyperopia and myopia are significantly higher in the anisometropic amblyopia, compared to anisometropic non-amblyopia.19
Significant hyperopia (>3.00D) was present in 55.6% of amblyopic children in the Sydney Pediatric Eye Disease Study (SPEDS). This study showed that amblyopia was strongly associated with anisometropia (OR, 27.82), hyperopia ≥2.00D (OR, 15.33), and astigmatism ≥1.00D (OR, 5.67), as in our report.20 Also among Singaporean Chinese children and Australian adults with unilateral amblyopia, anisometropic refractive error was the most common cause and in the bilateral amblyopia group, astigmatism was more common.21,22
Additionally, we found the mean SE for amblyopic eyes was 0.29D which was significantly more hyperopic than in the non-amblyopic eyes (−0.30D; P=0.001). Nevertheless, other studies like SPEDS reported higher mean hyperopia of about 3D.20 This can be explained by the higher age in our study, thus resulting in higher prevalence of myopia in our population compared to other studies. We found no sex difference between amblyopia and non-amblyopia groups, which is in agreement with a number of previous studies.22,23
We found significant associations between prematurity and low birth weight with amblyopia. Preterm and low birth weight children had 6–7 fold increase of having amblyopia in the present study. Our results are consistent with other studies that reported a higher risk of strabismus and amblyopia in preterm children compared with children born at full term.7–9 For example, Robaei et al. found 5-fold increase in risk of amblyopia in preterm children.7 Saunders et al.13 reported that premature birth carries a risk of abnormal refractive development because early emmetropisation process differed in preterm infants from that of the fullterm. In addition, there was an increased incidence of myopia and high hyperopia in low birth weight children.10
However, amblyopia was not associated with low birth weight, preterm birth, or maternal smoking during pregnancy in SPEDS.20 It has been speculated that prolonged exposure to illumination such as during treatment for jaundice may be implicated in the reduction of visual acuity.24 Alternatively, it has been proposed that prenatal endotoxin exposure through intrauterine infections, which can be linked to preterm delivery, may be harmful to the developing retina and optic nerve potentially impacting on visual development.25 Neurological damage, including ischemic brain lesions, may produce visual impairment in preterm children.26
In addition, we found dystocia in 13.7% of children with amblyopia and there was a significant association between dystocia with amblyopia. In other words, relative risk of amblyopia between those with and without dystocia was 4.65. Yi et al. found that 9.1% of amblyopic children had dystocia with natural labor.27
In conclusion, in clinical practice, presence of anisometropia, hyperopia, and prematurity (≤37 weeks) increased the risk for development of amblyopia. As a result of our findings and taking into account that our clinical data are not representative of the general population, we do recommend a full eye evaluation at the earliest possible timing with a regular clinical follow-up to be undertaken in the previously discussed high-risk infants and children to assess for presence and treatment of amblyopia.
There are several limitations to our study. First is the method used for measuring the visual acuity. We used single surround E optotypes, which can be quiet challenging for young children. Other studies including Amblyopia Treatment study28 protocol used isolated HOTV optotypes surround by crowding bars. However, there is no such standard for testing visual acuity in children in non-English countries like Iran. Another limiting factor was that we used two various methods of refraction (auto-refraction and manual retinoscopy), hence creating a confounding variable in inter-instrument's measurement for validity and reliability.
Conflicts of interestThe authors have no conflicts of interest to declare.